 |
年間契約型資訊服務
商品編碼
1563425
Top 200 Cell and Gene Facility Index:生物製藥製造商訂閱資料庫Top 200 Cell and Gene Facility Index and Biomanufacturers Subscription Database |
||||||
本資料庫資料庫為世界各地的生物製藥製造商提供細胞和基因療法的開發和製造服務,積極追蹤超過200個細胞/基因治療專用設施,唯一基於訂閱的資料庫。
全球細胞和基因治療市場規模,到2032年預計將達到約 822.4億美元。
資訊圖表
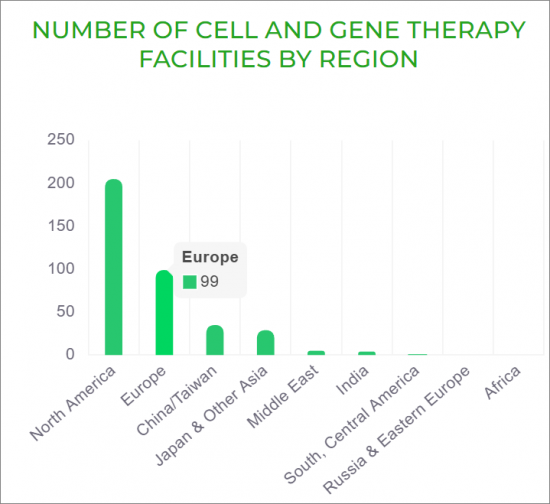
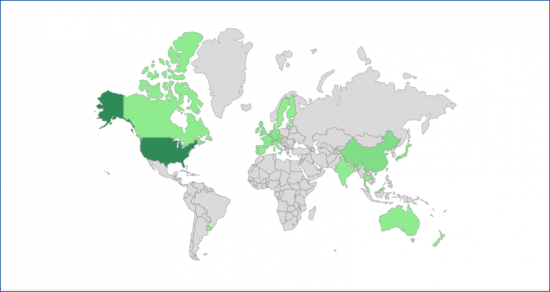
用於細胞和基因治療的生物製藥的世界製造集中度。
滾動瀏覽國家/地區名稱以查看生物過程強度和容量資料:
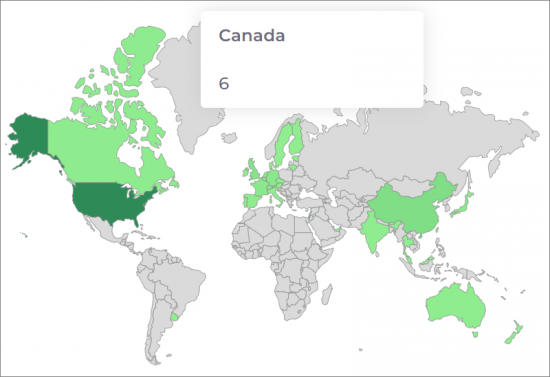
目前細胞/基因治療設施數:339
該資料庫不斷更新,包含基於過去 30年生物製藥和生命科學行業評估和基準的全面資訊和分析。
"Top 200 Cell and Gene Facility Index" 對每個細胞和基因治療 "專用" 設施進行排名,並提供生物製造區域和集群的定量區域分析。
- 生物反應器總容量
- 細胞和基因治療服務的專業知識
- 預計未來投產的生物製藥產能
- 商業和臨床生物製品的數量
- 收入預測
- 優勢與挑戰
- 技術和專業知識等
提供的內容
- 1年訂閱
- 生藥設施季刊通訊(免費)
- 可以進行儀表板搜尋和排序
- 定期資料庫更新
- 選項:存取即時分析師的查詢輸入
top200cellgene.com 的用途
- 確定理想的合作夥伴:建立細胞和基因治療(CT/GT)合作夥伴的關鍵網路
- 專業知識鑑定:細胞/基因治療、pDNA、mRNA
- 早期公司:贊助商可以確定潛在的製造合作夥伴。
- 設施位置:找到合格的員工並減少招募挑戰。
- 銷售區域分配:決定區域的位置以及人員配置方式。
- 市場競爭:決定競爭對手的優勢和劣勢。
- 不動產評估:根據當地吸引力評估設施。
- 人力資源和招募經理:尋找足夠數量的經過培訓且技術熟練的員工。
- 產業成長:了解產業在哪些領域成長最快。
- 新興地區:中國、印度、拉丁美洲和中東的CT/GT 設施。
- 設施排名:CT/GT 設施在技能、就業和生產能力方面的排名。
The ONLY Global Cell and Gene facilities database subscription that actively tracks over 200+ dedicated Cell and Gene Therapy facilities providing cell therapy and gene therapy development and manufacturing services for biopharmaceuticals.
Global cell and gene therapy market size is projected to reach approximately $82.24 billion by 2032.
CONCENTRATION OF GLOBAL CELL AND GENE THERAPY BIOPHARMACEUTICAL MANUFACTURING

Current Count of Cell and Gene Facilities: 339.
Based on 30 years of evaluating and benchmarking the biopharmaceutical and life sciences industry, this database is updated continually and includes comprehensive information and insights.
"Top200Cell and Gene Facility Index" ranks each 'dedicated' cell and gene therapy facility and provides regional quantitative analysis of biomanufacturing regions and clusters.
- Total bioreactor liter capacity
- Cell and Gene Therapy service expertise
- Future Biomanufacturing capacity coming online
- Number of commercial and clinical biological products manufactured
- Revenue estimates
- Strengths and Challenges
- Technologies and expertise, and much more
WHAT YOU'LL GET:
- Full 1 year Subscription
- Biofacilities Quarterly Newsletter (Free!)
- Dashboard: Searchable and sortable
- Regular database updates
- Optional: Access to live analysts for queries and input
USES FOR THE TOP200CellGene.COM
- Identify ideal partners: develop key networks with CT/GT partners
- Identify specialized expertise: cell or gene therapy, pDNA, mRNA
- Early-stage companies: sponsors can identify potential manufacturing partners
- Facility siting: Locate qualified employees; reduce hiring challenges
- Sales territory allocations: Determine where and how to staff territories
- Market Competition: Identify competitors' strengths and weaknesses
- Real estate valuation: For facilities, based on regional attractiveness
- HR and Recruiters: Looking for a critical mass of educated and skilled staff
- Industry Growth: Find where the industry is growing most rapidly
- Emerging Regions: CT/GT Facilities in China, India, Latin America, Middle East regions
- Facility Ranking: How do CT/GT facilities rank, in skills, employment, capacity













