 |
市場調查報告書
商品編碼
1424447
全球腎上腺素注射器市場:按類型、最終用戶、分銷管道、地區Global Epinephrine Autoinjector Market, By Type, By End User, By Distribution Channel, By Geography |
||||||
全球腎上腺素注射器市場預計將從2023年的 22 億美元增至2030年的 39.1 億美元,預測期內年複合成長率為 8.6%。
| 報告範圍 | 報告詳情 | ||
|---|---|---|---|
| 基準年 | 2022年 | 2023/2024年市場規模 | 22億美元 |
| 實際資料 | 2018-2021 | 預測期 | 2023-2030 |
| 預測期間2023/2024 至2030/2031年年複合成長率: | 8.60% | 2030/2031價值預測 | 39.1億美元 |
腎上腺素注射器是一次性自動注射器,用於緊急治療過敏反應,包括過敏反應。過敏反應是一種危及生命的疾病,由對某些食物、藥物、昆蟲叮咬或其他過敏原的極度過敏反應引起。過敏反應的症狀包括低血壓、口腔和喉嚨腫脹、皮疹、呼吸困難和休克。如果發生過敏反應,必須及時給予腎上腺素以減緩症狀的進展。與注射器相比,腎上腺素注射器可以快速自我注射腎上腺素,降低傷亡風險。
市場動態:
全球腎上腺素注射器市場的成長是由全球過敏反應盛行率上升所推動的。根據FAAN(食物過敏和過敏反應網路)公佈的資料,光是美國每年就有5萬例過敏反應病例,其中150至200例導致死亡。人們對與過敏相關的危及生命的疾病的認知不斷提高,以及先進腎上腺素注射器的可用性刺激市場需求。然而,這些設備的成本以及新興國家對替代治療方案的偏好限制了市場的成長。創新腎上腺素注射器的開發具有更高的易用性、準確性和更廣泛的應用範圍,預計將在未來幾年為製造商帶來利潤豐厚的機會。
由於技術進步、對過敏反應的認知不斷提高以及對使用者友善設計的日益重視,腎上腺素注射器市場見證顯著的趨勢。無針替代品和培訓的數位健康平台備受關注,提高了患者的舒適度和可及性。市場也在應對疫情後不斷變化的情況,要求醫療保健系統和供應鏈具有適應性。持續的技術創新、改進的設備功能和策略聯盟進一步塑造腎上腺素注射器市場的動態,以滿足快速變化的醫療環境中高風險個人和醫療保健提供者的需求。
本研究的主要特點
本報告對全球腎上腺素注射器市場進行了詳細分析,並提供了以2022年為基準年的預測期(2023-2030年)的市場規模和年複合成長率(CAGR%)。
揭示了各個細分市場的潛在商機,並為該市場說明了一系列有吸引力的投資提案。
提供了有關市場促進因素、限制因素、機會、新產品發布和核准、市場趨勢、區域前景、主要企業採取的競爭策略等的重要見解。
根據公司亮點、產品系列、主要亮點,實績和策略等參數對全球腎上腺素注射器市場的主要企業進行了介紹。
主要公司包括 Mylan、Teva Pharmaceutical、Impax Laboratories、Adamis Pharmaceuticals Corporation、Pfizer、ALK Abello、Lincoln Medical、Hospira、Sanofi 和 Kaleo。
從這份報告中獲得的見解將幫助行銷人員和公司負責人就未來的產品發布、升級、市場擴張和行銷策略做出明智的決策。
全球腎上腺素注射器市場報告迎合了該行業的各種相關人員,如投資者、供應商、產品製造商、經銷商、新進業者和財務分析師。
透過分析全球腎上腺素注射器市場時所使用的各種策略矩陣,促進相關人員的決策。
目錄
第1章 研究目的與前提
- 研究目標
- 先決條件
- 簡稱
第2章 市場展望
- 報告說明
- 市場定義和範圍
- 執行摘要
- Coherent Opportunity Map(COM)
第3章 市場動態、法規及趨勢分析
- 市場動態
- 影響分析
- 主要亮點
- 監管場景
- 服務交付組合
- PEST分析
- 波特的分析
- 併購場景
- 管道分析
第4章 全球腎上腺素注射器市場-冠狀病毒(COVID-19)大流行的影響
- 新型冠狀病毒感染疾病(COVID-19)的流行病學
- 供需面分析
- 經濟影響
第5章 全球腎上腺素注射器市場,依類型,2018-2030
- 15mg
- 3mg
- 5mg
第6章 全球腎上腺素注射器市場,依最終用戶分類,2018-2030年
- 醫院
- 診所
- 個人客戶
第7章 2018-2030年全球腎上腺素注射器市場,依通路
- 零售藥房
- 醫院藥房
- 網路藥房
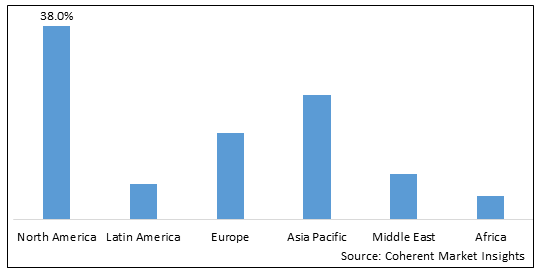
第8章 2018-2030年全球腎上腺素注射器市場,依地區
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 西班牙
- 法國
- 義大利
- 俄羅斯
- 其他歐洲國家
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- ASEAN
- 其他亞太地區
- 拉丁美洲
- 巴西
- 阿根廷
- 墨西哥
- 其他拉丁美洲
- 中東
- 海灣合作理事會國家
- 以色列
- 其他中東地區
- 非洲
- 北非
- 中部非洲
- 南非
第9章 競爭形勢
- Mylan
- Type Portfolio
- Teva Pharmaceutical
- Impax Laboratories
- Adamis Pharmaceuticals Corporation
- Pfizer
- ALK Abello
- Lincoln Medical
- Hospira
- Sanofi
- Kaleo
第10章
- 調查方法
- 關於出版商
Global epinephrine autoinjector market is expected to reach US$ 3.91 Bn by 2030, from US$ 2.20 Bn in 2023, exhibiting a CAGR of 8.6% during the forecast period.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2022 | Market Size in 2023/2024: | US$ 2.20 Bn |
| Historical Data for: | 2018 to 2021 | Forecast Period: | 2023 - 2030 |
| Forecast Period 2023/2024 to 2030/2031 CAGR: | 8.60% | 2030/2031 Value Projection: | US$ 3.91 Bn |

Epinephrine autoinjector is a single-use disposable automatic injection device that is intended for emergency treatment of allergic reactions including anaphylaxis. Anaphylaxis is a life-threatening condition that occur due to an extreme allergic reaction to certain foods, medicines, insects bites and other allergens. The symptoms of anaphylaxis include low blood pressure, swelling of the mouth or throat, rashes, breathing difficulty and shock. In case of anaphylaxis, epinephrine needs to be administered in a timely manner to curb the advancement of symptoms. Epinephrine autoinjectors enable rapid self-administration of epinephrine as compared to syringes, thereby, reducing the risk of casualty.
Market Dynamics:
Global epinephrine autoinjector market growth is driven by rising prevalence of anaphylaxis worldwide. According to the data published by FAAN (The Food Allergy and Anaphylaxis Network), 50,000 cases of anaphylaxis occur annually in the U.S. alone, out of which 150-200 result in death. Increased awareness regarding life-threatening conditions associated with allergies along with availability of advanced epinephrine autoinjector devices are fueling the market demand. However, cost of these devices and preference for alternative treatment options in developing nations limits the market growth. Development of innovative epinephrine autoinjectors with improved usability, accuracy and larger coverage area is expected to present lucrative opportunities for manufacturers in the coming years.
The epinephrine autoinjector market is witnessing notable trends, driven by advancements in technology, increased awareness of anaphylaxis, and a growing emphasis on user-friendly designs. Needle-free alternatives and digital health platforms for training are gaining prominence, enhancing patient comfort and accessibility. The market is also responding to the evolving landscape influenced by the pandemic, necessitating adaptability in healthcare systems and supply chains. Continuous innovation, improved device features, and strategic collaborations are further shaping the dynamics of the epinephrine autoinjector market, aiming to address the needs of at-risk individuals and healthcare providers in a rapidly changing healthcare environment.
Key features of the study:
This report provides in-depth analysis of the global epinephrine autoinjector market, and provides market size (US$ Bn) and compound annual growth rate (CAGR%) for the forecast period (2023-2030), considering 2022 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global epinephrine autoinjector market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include Mylan, Teva Pharmaceutical, Impax Laboratories, Adamis Pharmaceuticals Corporation, Pfizer, ALK Abello, Lincoln Medical, Hospira, Sanofi, and Kaleo
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
Global epinephrine autoinjector market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global epinephrine autoinjector market
Detailed Segmentation:
- Global Epinephrine Autoinjector Market, By Type
- 15mg
- 3mg
- 5mg
- Global Epinephrine Autoinjector Market, By End-User
- Hospitals
- Clinics
- Individual Customers
- Global Epinephrine Autoinjector Market, By Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
- Global Epinephrine Autoinjector Market, By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Global Epinephrine Autoinjector Market, Key Players
- Mylan
- Teva Pharmaceutical
- Impax Laboratories
- Adamis Pharmaceuticals Corporation
- Pfizer
- ALK Abello
- Lincoln Medical
- Hospira
- Sanofi
- kaleo
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Global Epinephrine Autoinjector Market, By Type
- Global Epinephrine Autoinjector Market, By End User
- Global Epinephrine Autoinjector Market, By Distribution Channel
- Global Epinephrine Autoinjector Market, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Growing prevalence of anaphylaxis
- Rising Prevalence of Food Allergies
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Service offering Portfolio
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
- Pipeline Analysis
4. Global Epinephrine Autoinjector Market- Impact of Coronavirus (COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Economic Impact
5. Global Epinephrine Autoinjector Market, By Type, 2018-2030, (US$ Bn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2019 - 2030
- Segment Trends
- 0.15mg
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- 0.3mg
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- 0.5mg
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
6. Global Epinephrine Autoinjector Market, By End User, 2018-2030, (US$ Bn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2019 - 2030
- Segment Trends
- Hospitals
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Clinics
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Individual Customers
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
7. Global Epinephrine Autoinjector Market, By Distribution Channel, 2018-2030, (US$ Bn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2019 - 2030
- Segment Trends
- Retail Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Hospital Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Online Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
8. Global Epinephrine Autoinjector Market, By Region, 2018-2030, (US$ Bn)
- Introduction
- Market Share Analysis, By Region, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, For Region, 2019 -2030
- Country Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030, (US$ Bn)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030, (US$ Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030, (US$ Bn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030, (US$ Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030, (US$ Bn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country/Region, 2018-2030, (US$ Bn)
- North Africa
- Central Africa
- South Africa
9. Competitive Landscape
- Mylan
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Teva Pharmaceutical
- Impax Laboratories
- Adamis Pharmaceuticals Corporation
- Pfizer
- ALK Abello
- Lincoln Medical
- Hospira
- Sanofi
- Kaleo
- Analyst Views
10. Section
- Research Methodology
- About us







