 |
市場調查報告書
商品編碼
1608701
兒科臨床試驗市場:依階段、依研究設計、依治療領域、按地區Pediatric Clinical Trials Market, By Phase, By Study Design, By Therapeutic Area (Respiratory Diseases, Cardiovascular Diseases, and Neuropsychiatric Conditions, Oncology, Diabetes, and Others ), By Geography |
||||||
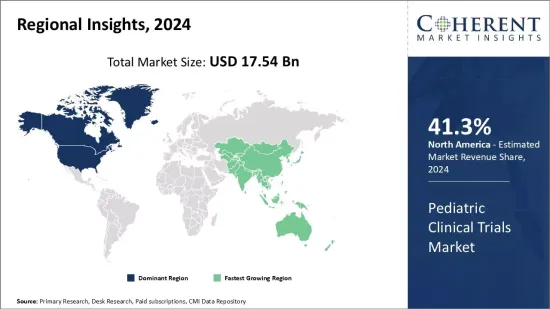
2024年兒科臨床試驗市場規模預計為175.4億美元,預計2031年將達333.1億美元,2024年至2031年複合年成長率為9.6%。
| 報告範圍 | 報告詳情 | ||
|---|---|---|---|
| 基準年 | 2023年 | 市場規模(2024年) | 175.4億美元 |
| 實際資料 | 2019-2023 | 預測期 | 2024-2031 |
| 預測期複合年成長率(2024-2031): | 9.60% | 預計金額(2031年) | 333.1億美元 |
近年來兒科臨床試驗市場經歷了顯著成長。兒童慢性病盛行率的不斷上升以及對新治療方案的需求不斷成長正在推動兒科臨床試驗產業的發展。此外,製藥公司正在增加對兒童藥物開發的投資。人們對罕見疾病的認知不斷提高,加上監管機構要求對兒科藥物進行臨床評估的努力正在擴大市場機會。然而,由於倫理問題、特定年齡的藥物代謝、招募困難以及對替代終點缺乏共識,進行兒科藥物的臨床試驗比成人試驗更加複雜和困難。然而,隨著新治療方法的出現以及公司不斷努力開發兒童友善藥物,兒科臨床試驗市場預計將在未來幾年強勁成長。
市場動態:
全球兒科臨床試驗市場的成長是由兒童慢性病盛行率上升、對新治療方法的需求不斷增加以及旨在簡化藥物開發流程的監管努力所推動的。根據世界衛生組織估計,2021年全球將有超過2億名兒童受到各種非傳染性疾病的影響。鑑於大量未滿足的需求,製藥公司活性化投資於兒科特異性治療方法的開發。美國食品藥物管理局(FDA) 2012 年法案等有利法規要求對兒科藥物進行上市前臨床評估,擴大了市場機會。然而,兒科臨床研究的複雜性和缺乏足夠的替代終點繼續對市場成長產生負面影響。與臨床試驗相關的高昂成本以及缺乏經驗豐富的臨床實驗也阻礙了更廣泛的市場採用。然而,產業相關人員和研究機構之間的持續合作,以新穎的方法解決問題,可能會支持未來的擴張。
本研究的主要特點
本報告對全球兒科臨床試驗市場進行了詳細分析,並列出了以2023年為基準年的預測期(2024-2031)的市場規模和複合年成長率。
它還揭示了各個細分市場的潛在商機,並說明了該市場有吸引力的投資提案矩陣。
它還提供了有關市場促進因素、限制因素、機會、新產品發布和核准、市場趨勢、區域前景、主要企業採取的競爭策略等的重要見解。
根據公司亮點、產品系列、主要亮點、績效和策略等參數,對全球兒科臨床試驗市場的主要企業進行概況分析。
主要公司包括 CSL Behring、賽諾菲、工業、Orchard Therapeutics plc.、Pharming Group NV、BioCryst Pharmaceuticals, Inc.、Ionis Pharmaceuticals, Inc.、Attune Pharmaceuticals、Arrowhead Pharmaceuticals, Inc.、Adverum Biotechnotical, Incceuticals、Arrowhead Pharmaceuticals, Inc.、Adverum Biotechnotical, Inc. 、CENTOGENE NV等
該報告的見解使負責人和公司經營團隊能夠就未來的產品發布、升級、市場擴張和行銷策略做出明智的決策。
全球兒科臨床試驗市場報告針對該行業的各個相關人員,如投資者、供應商、產品製造商、經銷商、新進業者和財務分析師。
透過用於分析全球兒科臨床試驗市場的各種策略矩陣,將有助於相關人員做出決策。
目錄
第1章 分析目的與前提
- 分析目的
- 先決條件
- 簡稱
第2章 市場展望
- 報告說明
- 市場定義和範圍
- 執行摘要
第3章市場動態、法規與趨勢分析
- 市場動態
- 影響分析
- 主要亮點
- 監管場景
- 產品發布/核准
- PEST分析
- 波特的分析
- 企業合併(M&A) 場景
- 流行病學
第 4 章 全球兒科臨床試驗市場:按階段分類,2019-2031 年(十億美元)
- 第一階段
- 第二階段
- 第三階段
- 第四階段
第5章 全球兒科臨床試驗市場:依研究設計,2019-2031 年(十億美元)
- 治療研究
- 觀察性研究
第6章兒科臨床試驗市場:依治療領域,2019-2031(十億美元)
- 呼吸系統疾病
- 心血管疾病
- 神經精神疾病
- 腫瘤學
- 糖尿病
- 其他(神經精神疾病等)
第7章 全球兒科臨床試驗市場:按地區分類,2019-2031 年(十億美元)
- 北美洲
- 拉丁美洲
- 歐洲
- 亞太地區
- 中東
- 非洲
第8章 競爭格局
- CSL Behring
- Sanofi
- Takeda Pharmaceutical Company Limited
- Orchard Therapeutics plc.
- Pharming Group NV
- BioCryst Pharmaceuticals, Inc.
- Ionis Pharmaceuticals, Inc.
- Attune Pharmaceuticals
- Arrowhead Pharmaceuticals, Inc.
- Adverum Biotechnologies, Inc.
- KalVista Pharmaceuticals
- CENTOGENE NV
第9章 分析師建議
- 命運之輪
- 分析師觀點
- 一致的機會圖
第10章 參考文獻與調查方法
- 參考
- 調查方法
- 關於出版商
The pediatric clinical trials market is estimated to be valued at USD 17.54 Bn in 2024 and is expected to reach USD 33.31 Bn by 2031, exhibiting a compound annual growth rate (CAGR) of 9.6% from 2024 to 2031.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2023 | Market Size in 2024: | 17.54 Bn |
| Historical Data for: | 2019 to 2023 | Forecast Period: | 2024 to 2031 |
| Forecast Period 2024 to 2031 CAGR: | 9.60% | 2031 Value Projection: | 33.31 Bn |

The pediatric clinical trials market is witnessing significant growth in recent times. Growing prevalence of chronic diseases among children and rising demand for new treatment options have boosted the pediatric clinical trials industry. Further, the market has witnessed rising investment from pharmaceutical companies for drug development targeting pediatric population. Growing awareness about rare diseases and various initiatives undertaken by regulatory bodies mandating clinical evaluation of drugs for pediatric use have augmented market opportunities. However, conducting clinical trials for pediatric drugs is more complex and challenging than adult trials due to ethical concerns, age specific drug metabolism, difficulty in recruitment, and lack of consensus over surrogate endpoints. Nevertheless, with emerging novel medical therapies and continuous efforts by players towards developing child-friendly drugs, the pediatric clinical trials market is poised to experience strong growth over the forthcoming years.
Market Dynamics:
The global pediatric clinical trials market growth is driven by the rising incidence of chronic diseases among children, growing demand for new treatment modalities, and initiatives undertaken by regulatory bodies to streamline drug development process. In 2021, as per WHO estimates, over 200 million children suffer from various non-communicable illnesses worldwide. High unmet clinical needs have encouraged pharmaceutical companies to boost investments towards developing specialized treatments for pediatric population. Favorable regulations such as the U.S. Food and Drug Administration's amendments Act 2012 have mandated pre-marketing clinical evaluation of drugs for pediatric use, thereby augmenting market opportunities. However, complexities involved in pediatric clinical research and lack of sufficient surrogate endpoints continue to negatively impact market growth. High costs associated with trials and inadequate number of experienced clinical investigators also impede broader market adoption. Nevertheless, continual collaborations between industry players and research organizations to address issues through novel approaches will support future expansion.
Key Features of the Study:
This report provides in-depth analysis of the global pediatric clinical trials market, and provides market size (US$ Bn) and compound annual growth rate (CAGR%) for the forecast period (2024-2031), considering 2023 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approvals, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global pediatric clinical trials market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include CSL Behring, Sanofi, Takeda Pharmaceutical Company Limited, Orchard Therapeutics plc., Pharming Group N.V., BioCryst Pharmaceuticals, Inc., Ionis Pharmaceuticals, Inc., Attune Pharmaceuticals, Arrowhead Pharmaceuticals, Inc., Adverum Biotechnologies, Inc., KalVista Pharmaceuticals, and CENTOGENE N.V.
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
The global pediatric clinical trials market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global pediatric clinical trials market
Market Segmentation
- Phase Insights (Revenue, USD Bn, 2019-2031)
- Phase I
- Phase II
- Phase III
- Phase IV
- Study Design Insights (Revenue, USD Bn, 2019-2031)
- Treatment Studies
- Observational Studies
- Therapeutic Area Insights (Revenue, USD Bn, 2019-2031)
- Respiratory Diseases
- Cardiovascular Diseases
- Neuropsychiatric Conditions
- Oncology
- Diabetes
- Others (Neuropsychiatric Conditions, etc.)
- Regional Insights (Revenue, USD Bn, 2019-2031)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- CSL Behring
- Sanofi
- Takeda Pharmaceutical Company Limited
- Orchard Therapeutics plc.
- Pharming Group N.V.
- BioCryst Pharmaceuticals, Inc.
- Ionis Pharmaceuticals, Inc.
- Attune Pharmaceuticals
- Arrowhead Pharmaceuticals, Inc.
- Adverum Biotechnologies, Inc.
- KalVista Pharmaceuticals
- CENTOGENE N.V.
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Pediatric Clinical Trials Market, By Phase
- Pediatric Clinical Trials Market, By Study Design
- Pediatric Clinical Trials Market, By Therapeutic Area
- Pediatric Clinical Trials Market, By Region
3. Market Dynamics, Regulations, And Trends Analysis
- Market Dynamics
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Product Launches/Approvals
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
- Epidemiology
4. Global Pediatric Clinical Trials Market, By Phase, 2019-2031, (USD Bn)
- Introduction
- Market Share Analysis, 2024 and 2031 (%)
- Y-o-Y Growth Analysis, 2020 - 2031
- Segment Trends
- Phase I
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
- Phase II
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
- Phase III
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
- Phase IV
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
5. Global Pediatric Clinical Trials Market, By Study Design, 2019-2031, (USD Bn)
- Introduction
- Market Share Analysis, 2024 and 2031 (%)
- Y-o-Y Growth Analysis, 2020 - 2031
- Segment Trends
- Treatment Studies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
- Observational Studies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
6. Pediatric Clinical Trials Market, By Therapeutic Area, 2019-2031, (USD Bn)
- Introduction
- Market Share Analysis, 2024 and 2031 (%)
- Y-o-Y Growth Analysis, 2020 - 2031
- Segment Trends
- Respiratory Diseases
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
- Cardiovascular Diseases
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
- Neuropsychiatric Conditions
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
- Oncology
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
- Diabetes
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
- Others (Neuropsychiatric Conditions, etc.)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2019-2031, (USD Bn)
7. Global Pediatric Clinical Trials Market, By Region, 2019 - 2031, Value (USD Bn)
- Introduction
- Market Share (%) Analysis, 2024,2027 & 2031, Value (USD Bn)
- Market Y-o-Y Growth Analysis (%), 2020 - 2031, Value (USD Bn)
- Regional Trends
- North America
- Introduction
- Market Size and Forecast, By Phase, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Country, 2019 - 2031, Value (USD Bn)
- U.S.
- Canada
- Latin America
- Introduction
- Market Size and Forecast, By Phase, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Country, 2019 - 2031, Value (USD Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Introduction
- Market Size and Forecast, By Phase, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Country, 2019 - 2031, Value (USD Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, By Phase, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Country, 2019 - 2031, Value (USD Bn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- Introduction
- Market Size and Forecast, By Phase, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Country, 2019 - 2031, Value (USD Bn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, By Phase, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2019 - 2031, Value (USD Bn)
- Market Size and Forecast, By Country/Sub-region, 2019 - 2031, Value (USD Bn)
- South Africa
- North Africa
- Central Africa
8. Competitive Landscape
- CSL Behring
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Sanofi
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Takeda Pharmaceutical Company Limited
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Orchard Therapeutics plc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Pharming Group N.V.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- BioCryst Pharmaceuticals, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Ionis Pharmaceuticals, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Attune Pharmaceuticals
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Arrowhead Pharmaceuticals, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Adverum Biotechnologies, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- KalVista Pharmaceuticals
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- CENTOGENE N.V.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
9. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
10. References and Research Methodology
- References
- Research Methodology
- About us




