|
市場調查報告書
商品編碼
1766327
腦積水分流術市場機會、成長動力、產業趨勢分析及 2025 - 2035 年預測Hydrocephalus Shunt Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2035 |
||||||
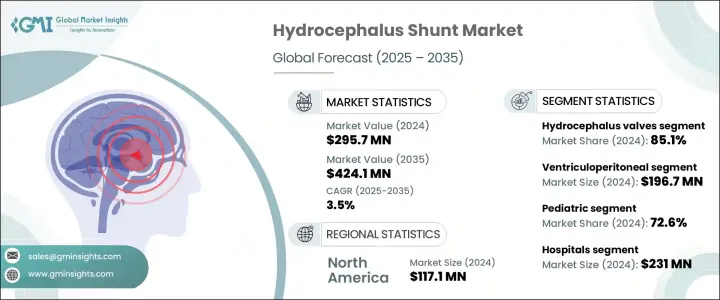
2024年,全球腦積水流器市場規模達2.957億美元,預計2035年將以3.5%的複合年成長率成長,達到4.241億美元。這一成長主要源於腦積水相關病例的不斷增加,這類病例通常與腦部外傷和腦腫瘤有關。隨著這些疾病的發生率不斷上升,對可靠治療方案的需求也日益成長。醫療技術的進步也對此市場產生了重大影響。可調式壓力分流閥、防虹吸系統以及生物相容性材料的應用等技術進步,正在改善患者的治療效果,同時最大限度地減少併發症。
智慧分流系統的引入,可以即時監測和調節腦脊髓液流量,進一步減少了手術修復的需求。 Integra LifeSciences Holdings、美敦力和貝朗等主要製造商持續投資研發和產品創新,以滿足不斷變化的臨床需求。腦積水分流器旨在將腦室中多餘的腦脊髓液轉移到身體其他部位進行吸收,從而防止顱內壓升高和潛在的神經損傷。醫學界越來越依賴這些系統來管理兒童和成人腦積水,這進一步鞏固了它們在現代神經外科實踐中的重要性。
2024年,腦積水瓣膜市場佔據主導地位,佔85.1%的市場。這一成長源於全球腦積水病例的不斷增加以及相關手術的激增。具有可程式控制和抗菌特性的創新瓣膜設計在降低併發症和修復率方面發揮著至關重要的作用。非侵入式壓力調節改善了患者管理,而許多地區老年人口的增加和出生率的提高也導致了腦積水患者人數的增加。外科醫生和醫療保健提供者擴大選擇能夠長期耐用且可個性化控制腦脊髓液引流的瓣膜,尤其是在專科醫療機構中。
2024年,腦室腹腔分流術的市場規模達1.967億美元。腦室腹腔分流術因其成本效益高、手術適應性強以及在各種臨床環境下均能取得一致療效,仍是治療腦積水的首選方法。與其他療法相比,腦室腹腔分流術是一種更經濟實惠且更廣泛採用的解決方案,尤其是在兒科護理和醫療預算有限的地區。兒童先天性腦積水病例的不斷增加,以及老年人對正常壓力腦積水的認知日益加深,正推動全球醫院擴大部署腦室腹腔分流術。
美國腦積水分流術市場規模預計到2034年將達到1.463億美元。在全國範圍內,腦積水分流術患者超過百萬,每年進行數萬例分流術,因此疾病仍然是一項重大的醫療挑戰。可程式分流術的創新、設備設計的改進以及更優的腦脊髓液調節方法推動了市場擴張。這些進展不僅改善了治療效果,也降低了修復手術的頻率。此外,持續的研發投入和先進產品的推出預計將提升該地區市場的發展軌跡。
腦積水分流器領域的主要市場參與者包括美敦力、貝朗、Integra LifeSciences Holdings、Sophysa、Luciole Medical、DESU、HLL Lifecare、Hpbio、Kaneka Medix、G. Surgiwear、Wellong Instruments Co. 和 Neutral Pharma。腦積水分流器市場的公司正專注於創新、研發投入和全球擴張,以鞏固其市場地位。
關鍵策略包括開發先進的可程式瓣膜、抗菌分流塗層以及具有即時監控功能的智慧分流系統。這些創新旨在最大限度地減少修復手術並提高病患安全性。各公司也正在與醫院和神經外科中心建立策略合作,以改善臨床回饋迴路並共同開發下一代設備。獲得監管部門的批准以及在新興經濟體建立強大的分銷網路也是公司的其他優先事項,這有助於公司進軍高成長地區。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 先天性腦積水盛行率不斷上升
- 人們對腦積水手術治療的認知不斷提高
- 腦積水治療的報銷範圍
- 老年人口不斷增加
- 產業陷阱與挑戰
- 新興國家缺乏進行複雜神經外科手術的基礎設施
- 腦積水流手術及後續護理費用高昂
- 市場機會
- 技術進步與智慧分流創新
- 人工智慧(AI)與先進診斷的融合
- 成長動力
- 成長潛力分析
- 監管格局
- 美國
- 歐洲
- 技術和創新格局
- 波特的分析
- PESTEL分析
- 未來市場趨勢
- 專利分析
第4章:競爭格局
- 介紹
- Medtronic
- Integra LifeSciences Holdings
- B. Braun
- 競爭市佔率分析
- 公司矩陣分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:依產品類型,2021 年至 2035 年
- 主要趨勢
- 腦積水瓣膜
- 調節壓力閥
- 固定壓力閥
- 腦積水導管
- 標準導管
- 抗生素導管
第6章:市場估計與預測:按程序類型,2021 年至 2035 年
- 主要趨勢
- 腦室腹腔
- 腰腹腔
- 心室心房
- 心室胸膜
第7章:市場估計與預測:依年齡層,2021 年至 2035 年
- 主要趨勢
- 兒科
- 成人
第8章:市場估計與預測:依最終用途,2021 年至 2035 年
- 主要趨勢
- 醫院
- 門診手術中心
第9章:市場估計與預測:按地區,2021 年至 2035 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 義大利
- 西班牙
- 荷蘭
- 亞太地區
- 日本
- 中國
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第10章:公司簡介
- B. Braun
- DESU
- G. Surgiwear
- HLL Lifecare
- Hpbio
- Integra LifeSciences Holdings
- Kaneka Medix
- Luciole Medical
- Medtronic
- Neutral Pharma
- Sophysa
- Wellong Instruments Co.
The Global Hydrocephalus Shunt Market was valued at USD 295.7 million in 2024 and is estimated to grow at a CAGR of 3.5% to reach USD 424.1 million by 2035. This growth is primarily driven by the rising number of cases related to hydrocephalus, frequently linked to traumatic brain injuries and brain tumors. As the prevalence of these conditions increases, so does the demand for reliable treatment solutions. Advances in medical technology have also significantly impacted this market. Developments such as adjustable pressure shunt valves, anti-siphon systems, and the use of biocompatible materials are enhancing patient outcomes while minimizing complications.

The introduction of smart shunt systems, which allow real-time cerebrospinal fluid monitoring and flow regulation, is further reducing the need for surgical revisions. Major manufacturers like Integra LifeSciences Holdings, Medtronic, and B. Braun continue to invest in research and product innovation to meet evolving clinical needs. Hydrocephalus shunts are designed to divert excess cerebrospinal fluid from the brain's ventricles to other areas of the body for absorption, preventing elevated intracranial pressure and potential neurological damage. The medical community increasingly relies on these systems for both pediatric and adult hydrocephalus management, further reinforcing their importance in modern neurosurgical practice.
The hydrocephalus valves segment led the market in 2024, capturing 85.1%. This growth stems from the increasing frequency of hydrocephalus cases and a surge in related surgeries worldwide. Innovative valve designs offering programmable control and antimicrobial properties are playing a crucial role in reducing complications and revision rates. Non-invasive pressure adjustments have improved patient management, while the rising number of elderly individuals and higher birth rates in many regions contribute to the growing hydrocephalus patient population. Surgeons and healthcare providers are increasingly choosing valves that offer long-term durability and personalized control over CSF drainage, especially in specialized medical facilities.
Ventriculoperitoneal shunts accounted for USD 196.7 million in 2024. VP shunting continues to be the preferred approach for treating hydrocephalus due to its cost-effectiveness, surgical adaptability, and consistent success across various clinical settings. Compared to alternative treatments, VP shunts offer a more affordable and widely adopted solution, particularly in pediatric care and regions with limited healthcare budgets. The rising number of cases involving congenital hydrocephalus in children and the growing recognition of normal pressure hydrocephalus among older adults are fueling the increased deployment of VP systems across hospitals globally.
United States Hydrocephalus Shunt Market is expected to reach USD 146.3 million by 2034. With over a million individuals affected nationwide and tens of thousands of shunt placements performed annually, the condition remains a significant medical challenge. Market expansion is supported by innovations in programmable shunts, improved device designs, and better cerebrospinal fluid regulation methods. These developments not only improve treatment outcomes but also decrease the frequency of revision procedures. Additionally, sustained investment in R&D and advanced product launches are expected to elevate the market's trajectory across the region.
Leading market participants in the Hydrocephalus Shunt Space include Medtronic, B. Braun, Integra LifeSciences Holdings, Sophysa, Luciole Medical, DESU, HLL Lifecare, Hpbio, Kaneka Medix, G. Surgiwear, Wellong Instruments Co., and Neutral Pharma. Companies operating in the hydrocephalus shunt market are focusing on innovation, R&D investment, and global expansion to reinforce their market positions.
Key strategies include developing advanced programmable valves, antimicrobial shunt coatings, and smart shunt systems with real-time monitoring features. These innovations aim to minimize revision surgeries and enhance patient safety. Firms are also entering strategic collaborations with hospitals and neurosurgery centers to improve clinical feedback loops and co-develop next-gen devices. Regulatory approvals and strong distribution networks across emerging economies are other priorities, enabling companies to tap into high-growth regions.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product type trends
- 2.2.3 Procedure type trends
- 2.2.4 Age group trends
- 2.2.5 End use trends
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of congenital hydrocephalus
- 3.2.1.2 Growing awareness towards surgical treatment of hydrocephalus
- 3.2.1.3 Availability of reimbursement coverage for hydrocephalus treatments
- 3.2.1.4 Increasing geriatric population
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Lack of infrastructure for performing complex neurosurgeries in emerging countries
- 3.2.2.2 High cost associated to hydrocephalus shunt surgery and follow-up care
- 3.2.3 Market opportunities
- 3.2.3.1 Technological Advancements and Smart Shunt Innovation
- 3.2.3.2 Integration of Artificial Intelligence (AI) and Advanced Diagnostics
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.3.1 By product type
- 3.3.2 By procedure type
- 3.3.3 By age group
- 3.3.4 By end use
- 3.4 Regulatory landscape
- 3.4.1 U.S.
- 3.4.2 Europe
- 3.5 Technology and innovation landscape
- 3.6 Porter's analysis
- 3.7 PESTEL analysis
- 3.8 Future market trends
- 3.9 Patent analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.1.1 Medtronic
- 4.1.2 Integra LifeSciences Holdings
- 4.1.3 B. Braun
- 4.2 Competitive market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 – 2035 ($ Mn)
- 5.1 Key trends
- 5.2 Hydrocephalus valves
- 5.2.1 Adjusted pressure valves
- 5.2.2 Fixed Pressure valves
- 5.3 Hydrocephalus catheter
- 5.3.1 Standard catheters
- 5.3.2 Antibiotic catheters
Chapter 6 Market Estimates and Forecast, By Procedure Type, 2021 – 2035 ($ Mn)
- 6.1 Key trends
- 6.2 Ventriculoperitoneal
- 6.3 Lumboperitoneal
- 6.4 Ventriculoatrial
- 6.5 Ventriculopleural
Chapter 7 Market Estimates and Forecast, By Age Group, 2021 – 2035 ($ Mn)
- 7.1 Key trends
- 7.2 Pediatric
- 7.3 Adult
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2035 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Ambulatory surgical centers
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2035 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Italy
- 9.3.5 Spain
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 Japan
- 9.4.2 China
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 B. Braun
- 10.2 DESU
- 10.3 G. Surgiwear
- 10.4 HLL Lifecare
- 10.5 Hpbio
- 10.6 Integra LifeSciences Holdings
- 10.7 Kaneka Medix
- 10.8 Luciole Medical
- 10.9 Medtronic
- 10.10 Neutral Pharma
- 10.11 Sophysa
- 10.12 Wellong Instruments Co.