 |
市場調查報告書
商品編碼
1665063
基於腫瘤的體內 CRO 市場機會、成長動力、產業趨勢分析與預測 2025 - 2034Oncology Based In-vivo CRO Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
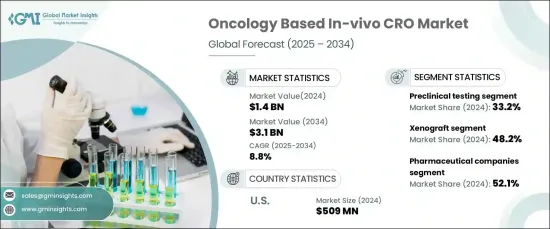
2024 年,全球基於腫瘤的體內 CRO 市場規模達到 14 億美元,預計 2025 年至 2034 年期間將以 8.8% 的強勁年複合成長率(CAGR) 成長。 這一顯著的市場擴張是由對免疫腫瘤療法的需求不斷成長、體內模型的不斷進步。
小型生物技術和製藥公司在推動腫瘤藥物發展方面發揮著至關重要的作用,從而加速了整體市場的成長。這些公司專注於創新治療方法,包括基因療法和基於抗體的藥物,旨在解決腫瘤學領域未滿足的醫療需求。他們在癌症研究方面的專業方法繼續推動對臨床前和體內測試服務的需求,進一步促進市場上升趨勢。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 14億美元 |
| 預測值 | 31億美元 |
| 複合年成長率 | 8.8% |
腫瘤體內 CRO 市場按服務類型細分,主要類別包括臨床前測試、功效測試、毒理學研究、藥物動力學等。 2024年臨床前測試佔據最大的市場佔有率,為33.2%。這種主導地位是由於臨床前測試在藥物開發中發揮的至關重要的作用,確保新療法在進入人體臨床試驗之前的安全性和有效性。美國 FDA 和 EMA 等監管機構要求臨床前測試是臨床試驗開始前的關鍵步驟。這些研究提供了有關藥物毒性、藥效學和治療效果的重要資料,使其成為腫瘤學研究過程中不可或缺的一部分。
市場也按模型類型細分,包括異種移植、同基因和其他模型。異種移植模型在 2024 年佔據市場主導地位,佔有 48.2% 的佔有率。這些模型因其能夠緊密模擬人類腫瘤生物學而受到廣泛青睞,為抗癌藥物的有效性提供了關鍵的見解。異種移植模型是將人類腫瘤組織植入免疫功能低下的小鼠體內,這可以準確模擬人類癌症的進展並增強藥物療效的評估。
在美國,腫瘤體內 CRO 市場在 2024 年的規模為 5.09 億美元,佔據區域主導地位。該國的市場領導地位可歸因於對腫瘤學研究的大量投資以及其製藥和生物技術領域的實力。由於許多公司專注於腫瘤藥物開發,美國嚴重依賴將臨床前和體內研究外包給 CRO。這一成長趨勢支持了市場的持續擴張,這得益於腫瘤治療的創新和對體內測試解決方案不斷成長的需求。
目錄
第 1 章:方法論與範圍
- 市場範圍和定義
- 研究設計
- 研究方法
- 資料收集方法
- 基礎估算與計算
- 基準年計算
- 市場估計的主要趨勢
- 預測模型
- 初步研究和驗證
- 主要來源
- 資料探勘來源
第 2 章:執行摘要
第 3 章:產業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 免疫腫瘤療法的需求不斷成長
- 體內模型的進展
- 精準腫瘤學投資不斷增加
- 小型生物技術和製藥公司批准的腫瘤藥物數量不斷增加
- 產業陷阱與挑戰
- 腫瘤藥物核准的嚴格監管要求
- 存在強力的替代方案
- 成長動力
- 成長潛力分析
- 監管格局
- 技術格局
- 專利分析
- 未來市場趨勢
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略展望
第 5 章:市場估計與預測:按服務,2021 年至 2034 年
- 主要趨勢
- 臨床前測試
- 功效測試
- 毒理學研究
- 藥物動力學
- 其他服務
第6章:市場估計與預測:依模型,2021 – 2034 年
- 主要趨勢
- 異種移植
- 患者來源的異種移植
- 細胞系來源的異種移植
- 其他異種移植
- 同源
- 其他型號
第 7 章:市場估計與預測:依最終用途,2021 年至 2034 年
- 主要趨勢
- 製藥公司
- 生技公司
- 其他最終用戶
第 8 章:市場估計與預測:按地區,2021 年至 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第9章:公司簡介
- Charles River Laboratories
- Crown Bioscience
- Eurofins Scientific
- Evotec SE
- ICON plc
- IMV
- Laboratory Corporation of America Holdings
- Medidata
- Merck KGaA
- OncoOne
- Pharmaron
- Taconic Biosciences
- The Jackson Laboratory
- Thermo Fisher Scientific
- WuXi AppTec
The Global Oncology Based In-Vivo CRO Market reached USD 1.4 billion in 2024 and is expected to grow at a robust compound annual growth rate (CAGR) of 8.8% from 2025 to 2034. This significant market expansion is being driven by the increasing demand for immuno-oncology therapies, ongoing advancements in in-vivo models, and a rise in oncology drug approvals from both biotechnology and pharmaceutical companies.

Small biotechnology and pharmaceutical companies are playing a crucial role in driving the development of oncology drugs, thus accelerating overall market growth. These companies focus on innovative treatments, including gene therapies and antibody-based drugs, which are designed to address unmet medical needs in the oncology space. Their specialized approach to cancer research continues to fuel the demand for preclinical and in-vivo testing services, further contributing to the market's upward trajectory.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.4 Billion |
| Forecast Value | $3.1 Billion |
| CAGR | 8.8% |
The oncology in-vivo CRO market is segmented by service type, with the primary categories being preclinical testing, efficacy testing, toxicology studies, pharmacokinetics, and others. Preclinical testing held the largest share of the market in 2024, accounting for 33.2%. This dominance is due to the vital role preclinical testing plays in drug development, ensuring the safety and efficacy of new therapies before they progress to human clinical trials. Regulatory authorities such as the U.S. FDA and EMA require preclinical testing as a critical step before clinical trials can commence. These studies provide essential data on drug toxicity, pharmacodynamics, and therapeutic effects, making them indispensable in the oncology research pipeline.
The market is also segmented by model type, including xenograft, syngeneic, and other models. Xenograft models led the market in 2024, holding a 48.2% share. These models are widely preferred due to their ability to closely mimic human tumor biology, offering crucial insights into the effectiveness of cancer drugs. Xenograft models involve implanting human tumor tissues into immunocompromised mice, which enables an accurate simulation of human cancer progression and enhances the evaluation of drug efficacy.
In the United States, the oncology in-vivo CRO market accounted for USD 509 million in 2024, dominating the regional landscape. The country's market leadership can be attributed to significant investments in oncology research and the strength of its pharmaceutical and biotechnology sectors. With numerous companies focused on oncology drug development, the U.S. heavily relies on outsourcing preclinical and in-vivo studies to CROs. This growing trend supports continued market expansion, driven by innovations in oncology therapies and a rising demand for in-vivo testing solutions.
Table of Contents
Chapter 1 Methodology & Scope
- 1.1 Market scope & definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates & calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing demand for immuno-oncology therapies
- 3.2.1.2 Advancements in in-vivo models
- 3.2.1.3 Growing investments in precision oncology
- 3.2.1.4 Growing oncology drug approvals from small biotechnology and pharmaceutical companies
- 3.2.2 Industry pitfalls & challenges
- 3.2.2.1 Stringent regulatory requirements for oncology drug approvals
- 3.2.2.2 Presence of strong alternatives
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Patent Analysis
- 3.7 Future market trends
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy outlook
Chapter 5 Market Estimates and Forecast, By Service, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Preclinical testing
- 5.3 Efficacy testing
- 5.4 Toxicology studies
- 5.5 Pharmacokinetics
- 5.6 Other services
Chapter 6 Market Estimates and Forecast, By Model, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Xenograft
- 6.2.1 Patient derived xenograft
- 6.2.2 Cell-line derived xenograft
- 6.2.3 Other xenografts
- 6.3 Syngeneic
- 6.4 Other models
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pharmaceutical companies
- 7.3 Biotechnology companies
- 7.4 Other end users
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Charles River Laboratories
- 9.2 Crown Bioscience
- 9.3 Eurofins Scientific
- 9.4 Evotec SE
- 9.5 ICON plc
- 9.6 IMV
- 9.7 Laboratory Corporation of America Holdings
- 9.8 Medidata
- 9.9 Merck KGaA
- 9.10 OncoOne
- 9.11 Pharmaron
- 9.12 Taconic Biosciences
- 9.13 The Jackson Laboratory
- 9.14 Thermo Fisher Scientific
- 9.15 WuXi AppTec













