 |
市場調查報告書
商品編碼
1684676
靜脈注射 (IV) 布洛芬市場機會、成長動力、產業趨勢分析與預測 2025 - 2034Intravenous (IV) Ibuprofen Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球靜脈注射布洛芬市值達到 76 億美元,預計 2025 年至 2034 年期間複合年成長率將達到驚人的 8.6%。這一成長軌跡反映了慢性和急性疾病(包括關節炎、心血管疾病和特定癌症)的盛行率上升,這些疾病通常需要靜脈注射布洛芬等有效的疼痛管理解決方案。此外,醫療保健基礎設施的進步、人們對非鴉片類疼痛管理替代品的認知的提高以及靜脈注射藥物配方的創新正在促進市場擴張。不同醫療環境中對快速、有效、安全的鎮痛解決方案的需求持續成長,使得靜脈注射布洛芬成為全球醫療保健提供者的首選。
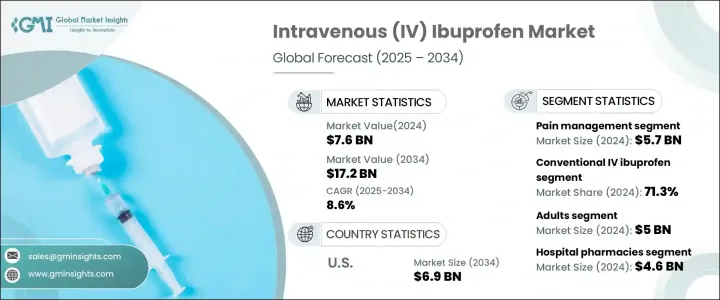
市場包括兩個主要產品類別:常規靜脈注射布洛芬和高濃度靜脈注射布洛芬。傳統靜脈注射布洛芬佔 2024 年 71.3% 的市場佔有率,由於其廣泛用於快速緩解疼痛,尤其是在急診室和術後場景,仍然是主導產品類型。其經過驗證的功效和安全性鞏固了其作為醫療專業人士信賴的解決方案的地位。由於其易於操作和對控制疼痛具有即時的效果,它成為了現代醫療保健中不可或缺的工具,推動了該領域的持續成長。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 76億美元 |
| 預測值 | 172億美元 |
| 複合年成長率 | 8.6% |
按年齡層分類,市場服務於兒童和成年人群,僅 2024 年成年人市場就將創收 50 億美元。人口老化,特別是患有關節炎和肌肉骨骼疾病等慢性疼痛疾病的人,對靜脈注射布洛芬的需求巨大。這種人口結構的變化凸顯了人們對快速止痛療法的日益依賴,尤其是當醫療保健提供者優先考慮在臨床和門診環境中盡量減少患者的不適時。隨著全球老年人口的不斷擴大,預計成年人口群體在預測期內仍將佔據主導地位。
在美國,靜脈注射布洛芬市場預計將大幅成長,到 2034 年將達到 69 億美元。推動這一成長的因素包括術後疼痛、肌肉骨骼疾病和其他與傷害相關的疾病的高盛行率。美國醫療保健系統注重有效的疼痛管理解決方案和龐大的患者群體,這增加了對靜脈注射布洛芬的需求。監管部門的認可進一步支持了這一趨勢。政府和衛生部門在通訊中強調了靜脈注射布洛芬在治療輕度至中度疼痛方面的功效,以及其與鴉片類鎮痛藥一起在治療重度疼痛方面的補充作用。這項監管支持增強了醫療保健提供者的信心,使靜脈注射布洛芬成為全國範圍內值得信賴的、緩解疼痛的首選方案。
目錄
第 1 章:方法論與範圍
- 市場範圍和定義
- 研究設計
- 研究方法
- 資料收集方法
- 基礎估算與計算
- 基準年計算
- 市場估計的主要趨勢
- 預測模型
- 初步研究和驗證
- 主要來源
- 資料探勘來源
第 2 章:執行摘要
第 3 章:產業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 術後疼痛發生率增加
- 非鴉片類疼痛管理的進展
- 急診和急救部門的入院人數不斷增加
- 靜脈給藥技術的進展
- 產業陷阱與挑戰
- 與口服替代品相比成本較高
- 潛在的副作用和禁忌症
- 成長動力
- 成長潛力分析
- 監管格局
- 技術格局
- 未來市場趨勢
- 差距分析
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第 5 章:市場估計與預測:按產品類型,2021 年至 2034 年
- 主要趨勢
- 常規靜脈注射布洛芬
- 高濃度靜脈注射布洛芬
第 6 章:市場估計與預測:按適應症,2021 年至 2034 年
- 主要趨勢
- 疼痛管理
- 發熱管理
第7章:市場估計與預測:依年齡層,2021 – 2034 年
- 主要趨勢
- 兒科
- 成年人
第 8 章:市場估計與預測:按配銷通路,2021 年至 2034 年
- 主要趨勢
- 醫院藥房
- 零售藥局
- 電子商務
第 9 章:市場估計與預測:按地區,2021 年至 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第10章:公司簡介
- AFT Pharmaceuticals
- Al Nabeel International
- Alveda Pharmaceuticals
- CSL Limited
- Cumberland Pharmaceuticals
- Fresenius SE & Co. KGaA
- Grifols
- Harbin Gloria Pharmaceuticals
- Hyloris Pharmaceuticals
- Laboratorios Valmorca
- Recordati
- Sandor Medicaids
- Soho Industri Pharmasi
- Teligent
- XGEN Pharmaceuticals
The Global Intravenous Ibuprofen Market reached a valuation of USD 7.6 billion in 2024 and is forecasted to expand at an impressive CAGR of 8.6% between 2025 and 2034. This growth trajectory reflects the rising prevalence of chronic and acute conditions, including arthritis, cardiovascular diseases, and specific cancers, which frequently necessitate effective pain management solutions like intravenous ibuprofen. Additionally, advancements in healthcare infrastructure, increasing awareness about non-opioid pain management alternatives, and innovations in intravenous drug formulations are bolstering market expansion. The demand for quick, effective, and safe analgesic solutions across diverse medical settings continues to grow, positioning intravenous ibuprofen as a preferred option for healthcare providers worldwide.

The market comprises two primary product categories: conventional intravenous ibuprofen and high-concentration intravenous ibuprofen. Conventional intravenous ibuprofen, accounting for 71.3% of the market share in 2024, remains the dominant product type due to its widespread use for rapid pain relief, especially in emergency rooms and post-surgical scenarios. Its proven efficacy and safety profile have solidified its place as a trusted solution among medical professionals. The ease of administration and immediate impact in managing pain make it an indispensable tool in modern healthcare, driving continued growth for this segment.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $7.6 Billion |
| Forecast Value | $17.2 Billion |
| CAGR | 8.6% |
Segmented by age group, the market serves both pediatric and adult populations, with adults generating USD 5 billion in 2024 alone. The aging population, particularly those dealing with chronic pain conditions such as arthritis and musculoskeletal disorders, is driving significant demand for intravenous ibuprofen. This demographic shift underscores the increasing reliance on fast-acting pain relief options, especially as healthcare providers prioritize minimizing patient discomfort in clinical and outpatient settings. With the elderly population expanding globally, the adult segment is expected to maintain its dominance over the forecast period.
In the United States, the intravenous ibuprofen market is projected to grow substantially, reaching USD 6.9 billion by 2034. Factors fueling this growth include the high prevalence of post-surgical pain, musculoskeletal disorders, and other injury-related conditions. The U.S. healthcare system's focus on effective pain management solutions and the large patient base amplifies the demand for intravenous ibuprofen. Regulatory endorsements further support this trend. Government and health authority communications have emphasized the efficacy of intravenous ibuprofen in treating mild to moderate pain and its complementary role alongside opioid analgesics for severe pain management. This regulatory backing enhances healthcare providers' confidence, making intravenous ibuprofen a trusted and preferred option in pain relief protocols across the nation.
Table of Contents
Chapter 1 Methodology & Scope
- 1.1 Market scope & definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates & calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° Synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing incidence of post-operative pain
- 3.2.1.2 Advancements in non-opioid pain management
- 3.2.1.3 Rising admissions in emergency and acute care departments
- 3.2.1.4 Advances in intravenous drug delivery technologies
- 3.2.2 Industry pitfalls & challenges
- 3.2.2.1 High cost compared to oral alternatives
- 3.2.2.2 Potential side effects and contraindications
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Conventional IV ibuprofen
- 5.3 High-concentration IV ibuprofen
Chapter 6 Market Estimates and Forecast, By Indication, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Pain management
- 6.3 Fever management
Chapter 7 Market Estimates and Forecast, By Age Group, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pediatrics
- 7.3 Adults
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 E-commerce
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 AFT Pharmaceuticals
- 10.2 Al Nabeel International
- 10.3 Alveda Pharmaceuticals
- 10.4 CSL Limited
- 10.5 Cumberland Pharmaceuticals
- 10.6 Fresenius SE & Co. KGaA
- 10.7 Grifols
- 10.8 Harbin Gloria Pharmaceuticals
- 10.9 Hyloris Pharmaceuticals
- 10.10 Laboratorios Valmorca
- 10.11 Recordati
- 10.12 Sandor Medicaids
- 10.13 Soho Industri Pharmasi
- 10.14 Teligent
- 10.15 XGEN Pharmaceuticals







