 |
市場調查報告書
商品編碼
1698253
磺達肝癸鈉市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Fondaparinux Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球磺達肝癸鈉市值為 6.919 億美元,預計 2025 年至 2034 年期間的複合年成長率為 6.8%。市場擴張的動力在於深部靜脈栓塞 (DVT) 和肺栓塞 (PE) 等血栓栓塞性疾病發生率的增加,以及對有效抗凝血療法的偏好日益成長。磺達肝癸鈉是一種合成抗凝血劑,在預防和治療血栓方面發揮至關重要的作用,它具有針對性的作用機制,並降低了肝素誘發併發症的風險。包括骨科手術在內的外科手術數量的不斷增加,進一步推動了對抗凝血治療的需求,鞏固了磺達肝癸鈉作為該領域主要參與者的地位。醫療保健基礎設施的擴張和具有成本效益的治療方案的增加繼續支持市場成長。此外,有利的監管政策和對先進抗凝血療法的推動加強了磺達肝癸鈉在住院和門診環境中的應用。
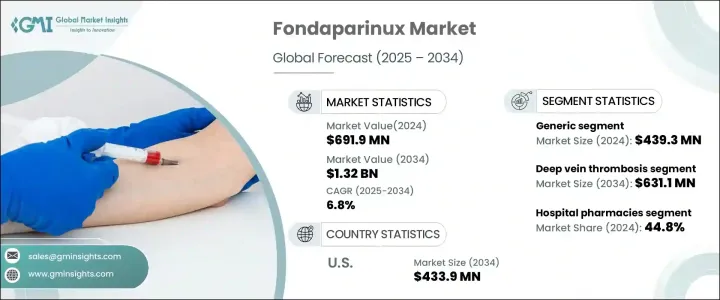
磺達肝癸鈉市場分為品牌藥和學名藥,其中學名藥由於價格低廉、使用廣泛而佔據主導地位。 2024 年,學名藥部門創造了 4.393 億美元的收入,凸顯了其強大的市場地位。隨著醫療保健提供者和患者尋求品牌藥物的經濟有效的替代品,特別是在需要長期抗凝血治療的情況下,對仿製磺達肝癸鈉的需求持續上升。隨著越來越多的患者需要持續預防血栓,學名藥提供了一種方便且經濟實惠的解決方案,推動了醫院、診所和家庭護理機構的採用。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 6.919億美元 |
| 預測值 | 13.2億美元 |
| 複合年成長率 | 6.8% |
依應用細分顯示,磺達肝癸鈉廣泛用於治療深部靜脈栓塞 (DVT)、肺栓塞 (PE) 和急性冠狀動脈症候群 (ACS)。 2024 年,DVT 領域佔據了 47.8% 的市場佔有率,反映出全球 DVT 病例的盛行率不斷上升。老年人和患有慢性疾病的人面臨更高的血栓形成風險,因此有效的抗凝血治療至關重要。由於其療效可靠、藥物動力學可預測,且與某些傳統抗凝血劑不同,不需要常規血液監測,因此磺達肝癸鈉仍是一種首選治療方法。
2024 年,美國磺達肝癸鈉市場產值達 2.443 億美元,預計到 2034 年將達到 4.339 億美元。促成這一擴張的因素有很多,包括大量的骨科手術、人口老化以及需要抗凝血治療的重大疾病盛行率上升。先進治療方案的日益普及以及對更安全、更有效的抗凝血劑的需求繼續推動磺達肝癸鈉在美國市場的地位,加強其在現代醫療保健中的作用。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 血栓栓塞性疾病發生率上升
- 髖關節和膝關節移植手術增加
- 全球老齡人口激增
- 產業陷阱與挑戰
- 與磺達肝癸鈉相關的副作用
- 成長動力
- 成長潛力分析
- 監管格局
- 管道分析
- 未來市場趨勢
- 差距分析
- 波特的分析
- PESTEL 分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:依產品類型,2021 年至 2034 年
- 主要趨勢
- 品牌
- 通用的
第6章:市場估計與預測:按應用,2021 年至 2034 年
- 主要趨勢
- 深層靜脈栓塞
- 肺栓塞
- 急性冠狀動脈綜合症
- 其他應用
第7章:市場估計與預測:按配銷通路,2021 年至 2034 年
- 主要趨勢
- 醫院藥房
- 零售藥局
- 網路藥局
第8章:市場估計與預測:按地區,2021 年至 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 沙烏地阿拉伯
- 南非
- 阿拉伯聯合大公國
第9章:公司簡介
- Abbott Laboratories
- Alchemia
- Apotex
- Aurobindo
- Dr. Reddy's Laboratories
- Jiangsu Hengrui Medicine
- Lupin Pharmaceuticals
- Sandoz
- ScinoPharm Taiwan
- Viatris
The Global Fondaparinux Market was valued at USD 691.9 million in 2024 and is projected to expand at a CAGR of 6.8% between 2025 and 2034. The market expansion is fueled by the increasing incidence of thromboembolic disorders such as deep vein thrombosis (DVT) and pulmonary embolism (PE), as well as the growing preference for effective anticoagulation therapies. Fondaparinux, a synthetic anticoagulant, plays a vital role in preventing and treating blood clots, offering a targeted mechanism of action with a lower risk of heparin-induced complications. The rising number of surgical procedures, including orthopedic surgeries, further propels the demand for anticoagulant treatments, solidifying fondaparinux's position as a key player in this segment. The expansion of healthcare infrastructure and the increased availability of cost-effective treatment options continue to support market growth. Additionally, favorable regulatory policies and the push for advanced anticoagulant therapies reinforce fondaparinux's adoption across inpatient and outpatient settings.

The fondaparinux market is divided into branded and generic versions, with the generic segment maintaining a dominant position due to its affordability and widespread use. In 2024, the generic segment generated USD 439.3 million in revenue, highlighting its strong market presence. The demand for generic fondaparinux continues to rise as healthcare providers and patients seek cost-effective alternatives to branded drugs, particularly in cases requiring long-term anticoagulation therapy. With an increasing number of patients requiring continued blood clot prevention, generic formulations provide an accessible and budget-friendly solution, driving adoption across hospitals, clinics, and home care settings.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $691.9 Million |
| Forecast Value | $1.32 Billion |
| CAGR | 6.8% |
Segmentation by application reveals that fondaparinux is widely used for treating deep vein thrombosis (DVT), pulmonary embolism (PE), and acute coronary syndrome (ACS). In 2024, the DVT segment accounted for 47.8% of the market, reflecting the growing prevalence of DVT cases worldwide. Older adults and individuals with chronic health conditions face higher risks of clot formation, making effective anticoagulation therapy essential. Fondaparinux remains a preferred treatment due to its reliable efficacy, predictable pharmacokinetics, and the advantage of not requiring routine blood monitoring, unlike certain traditional anticoagulants.
The U.S. fondaparinux market generated USD 244.3 million in 2024 and is projected to reach USD 433.9 million by 2034. Several factors contribute to this expansion, including a high volume of orthopedic procedures, an aging population, and a rising prevalence of critical illnesses that necessitate anticoagulation therapy. The increasing adoption of advanced treatment protocols and the demand for safer, more efficient anticoagulants continue to drive fondaparinux's market presence in the U.S., reinforcing its role in modern healthcare.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising incidence of thromboembolic diseases
- 3.2.1.2 Increase in hip and knee transplant surgeries
- 3.2.1.3 Surge in aging population worldwide
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Side effects associated with fondaparinux
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Pipeline analysis
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Branded
- 5.3 Generic
Chapter 6 Market Estimates and Forecast, By Application, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Deep vein thrombosis
- 6.3 Pulmonary embolism
- 6.4 Acute coronary syndrome
- 6.5 Other applications
Chapter 7 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospital pharmacies
- 7.3 Retail pharmacies
- 7.4 Online pharmacies
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 Saudi Arabia
- 8.6.2 South Africa
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott Laboratories
- 9.2 Alchemia
- 9.3 Apotex
- 9.4 Aurobindo
- 9.5 Dr. Reddy’s Laboratories
- 9.6 Jiangsu Hengrui Medicine
- 9.7 Lupin Pharmaceuticals
- 9.8 Sandoz
- 9.9 ScinoPharm Taiwan
- 9.10 Viatris






