 |
市場調查報告書
商品編碼
1699401
植入式醫療器材市場機會、成長動力、產業趨勢分析及2025-2034年預測Implantable Medical Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025-2034 |
||||||
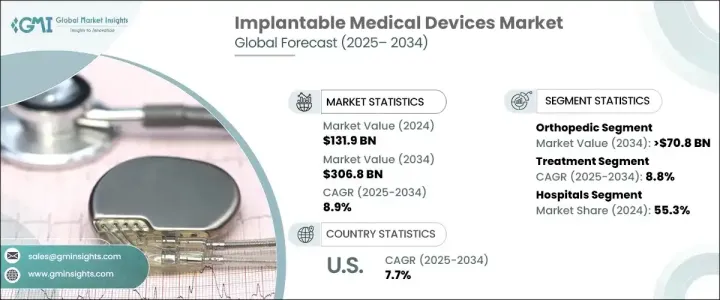
2024 年全球植入式醫療器材市場價值為 1,319 億美元,預估 2025 年至 2034 年期間的複合年成長率為 8.9%。這一成長是由心血管疾病、神經系統疾病和骨科問題等慢性疾病的日益流行所推動的,所有這些疾病都需要先進的植入式解決方案。隨著全球人口老化,對起搏器、神經刺激器、骨科植入物和其他改善生活品質的設備的需求持續激增。老年人口仍然是市場擴張的重要推動力,因為他們更容易患上與年齡相關的疾病和慢性病。
技術進步正在進一步重塑植入式醫療器材產業,生物相容性材料、無線技術和智慧植入物的創新增強了這些設備的功能和耐用性。微創手術的興起和患者對持久解決方案的偏好也推動了該技術的採用。人工智慧 (AI) 與醫療物聯網 (IoMT) 的融合正在創造新的成長途徑,現在的設備能夠即時健康監測和資料傳輸,從而更好地管理患者。市場上 3D 列印植入物的數量也在激增,從而實現了更高程度的客製化並改善了手術效果。隨著全球醫療保健支出的增加以及下一代植入物的法規核准放寬,製造商正專注於研發,以推出更有效率、以患者為中心的設備。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 1319億美元 |
| 預測值 | 3068億美元 |
| 複合年成長率 | 8.9% |
市場分為不同的類別,其中骨科器械預計將大幅成長。預計骨科領域將以 5.7% 的複合年成長率成長,到 2034 年將達到 708 億美元。骨質疏鬆症、骨關節炎和骨折病例的增加導致對骨科植入物的需求增加。關節置換手術的數量不斷增加,尤其是髖關節、膝關節和脊椎植入手術,進一步加速了市場擴張。此外,由於道路交通事故和運動相關事故造成的創傷發生率不斷上升,推動了全球先進骨科解決方案的採用。
植入式醫療器材產業也分為治療設備和診斷設備。治療領域涵蓋用於治療目的的設備,預計將以 8.8% 的複合年成長率成長,到 2034 年達到 3032 億美元。糖尿病和心血管疾病等生活方式相關疾病的盛行率日益上升,推動了對創新耐用的植入式解決方案的需求。這些設備顯著改善了患者的治療效果和生活品質,從而得到了廣泛的應用。
美國植入式醫療器材市場規模在 2024 年將達到 548 億美元,預計 2025 年至 2034 年的複合年成長率將達到 7.7%。美國在技術進步方面仍然處於全球領先地位,無線充電、小型化和人工智慧驅動的植入物方面的創新正在徹底改變整個產業。提供即時資料和個人化治療方案的智慧植入物正在受到醫療保健提供者的青睞。此外,3D列印技術正在改變個人化植入物的生產,並提高手術的精確度。憑藉持續的研發投入和強大的醫療保健基礎設施,美國將繼續處於植入式醫療設備創新的前沿。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 全球慢性病發生率不斷上升
- 器官捐贈者稀缺
- 已開發國家的技術進步
- 已開發國家政府對植入式醫療器材的資助不斷增加
- 越來越重視微電子和植入式感測器的發展
- 擴大採用生物材料來獲得更好的生物相容性和療效
- 產業陷阱與挑戰
- 設備成本高
- 有源植入式醫療器材的嚴格規定
- 術後併發症發生率較高
- 大量植入式醫療器材召回
- 成長動力
- 成長潛力分析
- 監管格局
- 技術格局
- 差距分析
- 波特的分析
- PESTEL分析
- 未來市場趨勢
- 價值鏈分析
- 植入性醫療器材安全概述
第4章:競爭格局
- 介紹
- 公司矩陣分析
- 公司市佔率分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:按產品,2021 - 2034 年
- 主要趨勢
- 骨科
- 關節重建
- 脊椎裝置
- 創傷固定裝置
- 其他骨科產品
- 心血管
- 支架
- 植入式心臟去顫器(ICD)
- 起搏器
- 心臟再同步治療(CRT)
- 心室輔助裝置
- 植入式心臟監測儀(ICM)
- 其他心血管產品
- 牙科
- 牙冠和基台
- 牙種植體
- 其他牙科產品
- 神經病學
- 深部腦部刺激器
- 其他神經病學產品
- 眼科
- 人工水晶體和青光眼植入物
- 其他眼科產品
- 整型手術
- 乳房植入物
- 臀肌植入物
- 其他產品
第6章:市場估計與預測:按類型,2021 - 2034 年
- 主要趨勢
- 治療
- 診斷
第7章:市場估計與預測:依設備性質,2021 - 2034 年
- 主要趨勢
- 靜態/非主動/被動
- 積極的
第8章:市場估計與預測:依最終用途,2021 - 2034 年
- 主要趨勢
- 醫院
- 門診手術中心
- 多專科中心
- 診所
- 其他最終用途
第9章:市場估計與預測:按地區,2021 - 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第10章:公司簡介
- Abbott
- Advanced Bionics
- Alcon
- Allergan
- BAUSCH + LOMB
- BIOTRONIK
- Boston Scientific
- Cochlear
- Demant
- GORE
- HENRY SCHEIN
- Johnson & Johnson
- MED-EL
- Medtronic
- MicroPort
- mindray
- POLYTECH
- smith & nephew
- stryker
- ZIMMER BIOMET
The Global Implantable Medical Devices Market was valued at USD 131.9 billion in 2024 and is projected to grow at a CAGR of 8.9% between 2025 and 2034. This growth is fueled by the increasing prevalence of chronic conditions such as cardiovascular diseases, neurological disorders, and orthopedic issues, all of which require advanced implantable solutions. As the global population ages, the demand for pacemakers, neurostimulators, orthopedic implants, and other life-enhancing devices continues to surge. The elderly demographic remains a significant driver of market expansion, given their higher susceptibility to age-related ailments and chronic conditions.

Technological advancements are further reshaping the implantable medical devices industry, with innovations in biocompatible materials, wireless technology, and smart implants enhancing the functionality and durability of these devices. The rise of minimally invasive procedures and patient preference for long-lasting solutions are also propelling adoption. The integration of artificial intelligence (AI) and the Internet of Medical Things (IoMT) is creating new growth avenues, with devices now capable of real-time health monitoring and data transmission for better patient management. The market is also seeing a surge in 3D-printed implants, allowing for greater customization and improved surgical outcomes. With healthcare spending increasing globally and regulatory approvals easing for next-generation implants, manufacturers are focusing on research and development to introduce more efficient and patient-centric devices.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $131.9 Billion |
| Forecast Value | $ 306.8 Billion |
| CAGR | 8.9% |
The market is segmented into various categories, with orthopedic devices expected to witness substantial growth. The orthopedic segment is projected to grow at a CAGR of 5.7%, reaching USD 70.8 billion by 2034. Rising cases of osteoporosis, osteoarthritis, and fractures are contributing to the higher demand for orthopedic implants. The increasing number of joint replacement surgeries, particularly hip, knee, and spinal implants, is further accelerating market expansion. Additionally, the growing incidence of traumatic injuries due to road accidents and sports-related mishaps is driving the adoption of advanced orthopedic solutions worldwide.
The implantable medical devices industry is also divided into treatment and diagnostic devices. The treatment segment, which encompasses devices used for therapeutic purposes, is anticipated to grow at a CAGR of 8.8%, reaching USD 303.2 billion by 2034. The increasing prevalence of lifestyle-related diseases, such as diabetes and cardiovascular disorders, is driving demand for innovative and durable implantable solutions. These devices significantly enhance patient outcomes and quality of life, leading to widespread adoption.
U.S. Implantable Medical Devices Market, valued at USD 54.8 billion in 2024, is expected to expand at a CAGR of 7.7% from 2025 to 2034. The country remains a global leader in technological advancements, with innovations in wireless charging, miniaturization, and AI-driven implants revolutionizing the industry. Smart implants that provide real-time data and personalized treatment options are gaining traction among healthcare providers. Furthermore, 3D printing technology is transforming the production of personalized implants, improving precision in surgical procedures. With continuous R&D investments and a robust healthcare infrastructure, the U.S. is poised to remain at the forefront of implantable medical device innovations.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing incidence of chronic diseases across the globe
- 3.2.1.2 Scarcity of organ donors
- 3.2.1.3 Technological advancement in developed nations
- 3.2.1.4 Rising government funding for implantable medical devices in developed countries
- 3.2.1.5 Increasing focus on the development of microelectronics and implantable sensors
- 3.2.1.6 Growing adoption of biomaterials for better biocompatibility and outcomes
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of devices
- 3.2.2.2 Stringent regulations for active implantable medical devices
- 3.2.2.3 Considerable rate of post-procedural complications
- 3.2.2.4 High number of recalls of implantable medical devices
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technology landscape
- 3.6 Gap analysis
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
- 3.9 Future market trends
- 3.10 Value chain analysis
- 3.11 Overview on implantable medical device security
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Orthopedic
- 5.2.1 Joint reconstruction
- 5.2.2 Spinal devices
- 5.2.3 Trauma fixation devices
- 5.2.4 Other orthopedic products
- 5.3 Cardiovascular
- 5.3.1 Stents
- 5.3.2 Implantable cardiac defibrillators (ICDs)
- 5.3.3 Pacemaker
- 5.3.4 Cardiac resynchronization therapy (CRT)
- 5.3.5 Ventricular assist devices
- 5.3.6 Implantable cardiac monitors (ICM)
- 5.3.7 Other cardiovascular products
- 5.4 Dental
- 5.4.1 Dental crowns and abutment
- 5.4.2 Dental implants
- 5.4.3 Other dental products
- 5.5 Neurology
- 5.5.1 Deep brain stimulators
- 5.5.2 Other neurology products
- 5.6 Ophthalmology
- 5.6.1 Intraocular lenses and glaucoma implants
- 5.6.2 Other ophthalmology products
- 5.7 Plastic surgery
- 5.7.1 Breast implants
- 5.7.2 Gluteal implants
- 5.8 Other products
Chapter 6 Market Estimates and Forecast, By Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Treatment
- 6.3 Diagnostic
Chapter 7 Market Estimates and Forecast, By Nature of Device, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Static/Non-active/Passive
- 7.3 Active
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Ambulatory surgical centers
- 8.4 Multi-specialty centers
- 8.5 Clinics
- 8.6 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott
- 10.2 Advanced Bionics
- 10.3 Alcon
- 10.4 Allergan
- 10.5 BAUSCH + LOMB
- 10.6 BIOTRONIK
- 10.7 Boston Scientific
- 10.8 Cochlear
- 10.9 Demant
- 10.10 GORE
- 10.11 HENRY SCHEIN
- 10.12 Johnson & Johnson
- 10.13 MED-EL
- 10.14 Medtronic
- 10.15 MicroPort
- 10.16 mindray
- 10.17 POLYTECH
- 10.18 smith & nephew
- 10.19 stryker
- 10.20 ZIMMER BIOMET







