 |
市場調查報告書
商品編碼
1648561
人為因素和可用性工程服務市場:依步驟、研究方法類型、測試的醫療器材類別、地區和主要參與者:產業趨勢和2035年全球預測Human Factors and Usability Engineering Services Market by Steps Involved, Type of Research Method, Class of Medical Device Tested, Geographical Regions and Leading Players: Industry Trends and Global Forecasts, Till 2035 |
||||||
全球人為因素和可用性工程服務市場規模預計將從目前的9.4億美元成長到2035年的19.7億美元,預測期內的年複合成長率為 6.9%。
多年來,人為因素和可用性工程一直是各類醫療設備開發中不可或缺的一部分。因此,開發人員越來越依賴服務提供者對其設備進行人為因素研究,以提高其安全性、有效性和使用者體驗。隨著醫療設備變得越來越複雜,必須致力於原型設計和使用者介面設計,以盡量減少與其使用相關的潛在風險。此外,監管機構(尤其是美國食品藥物管理局)日益要求將人為因素納入醫療器材的設計和評估中。這些機構開始採用系統化的方法來評估製造商對可用性工程標準的遵守情況。
醫療器材領域對人為因素和可用性工程的重視反映了對病人安全和設備可用性的更廣泛的承諾。這增加了在整個產品生命週期中(而不是僅在開發的後期階段)對設備的人為因素和可用性工程的需求。這使得設備開發人員能夠及時對設備開發過程進行微調,以確保用戶滿意度並消除風險。隨著對創新和個人化醫療設備的需求不斷成長,人為因素和可用性工程服務的外包預計在不久的將來會增加。
目前,超過 210 家公司聲稱擁有為各種醫療設備提供人為因素和可用性工程服務所需的專業知識。
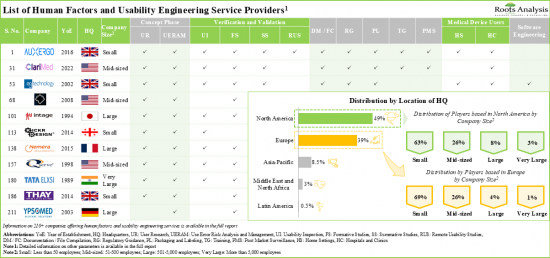
超過95%的服務供應商提供產品設計和開發相關服務,其中大多數支援診斷設備測試。為了在這個競爭激烈的行業中保持領先地位,公司不斷提升其能力以增強其人為因素工程服務組合。
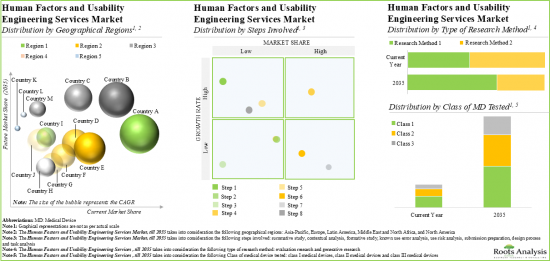
本報告概述了全球人為因素和可用性工程服務市場,並提供了有關市場趨勢的詳細資訊,包括分步趨勢、研究方法類型、測試的醫療器材類別、地區、主要公司以及市場參與者的概況。
目錄
第1章 簡介
第2章 研究方法
第3章 市場動態
第4章 經濟及其他專案特定考量
第5章 執行摘要
第6章 簡介
第7章 人為因素與可用性工程監理指南
第8章 市場狀況
第9章 企業競爭力分析
第10章 公司簡介:北美服務供應商
- 章節概述
- ClariMed
- Aptar Digital Health
- Bold Insight
- Greenlight Guru
- Key Winning Strategies
第11章 公司簡介:歐洲服務提供者
- 章節概述
- Bayoomed
- AYES
- Comate
- Key Winning Strategies
第12章 公司簡介:亞太地區服務提供者
- 章節概述
- Hydrix
- D+I
- Invetech
- Johari Digital
- Key Winning Strategies
第13章 合併與收購
第14章 人為因素和可用性工程過程相關的成本影響
第15章 案例研究:醫療器材召回以及人為因素和可用性工程的作用
第16章 人為因素與可用性工程服務市場:新興大趨勢
第17章 市場影響分析
- 章節概述
- 市場驅動因素
- 市場限制
- 市場機會
- 市場挑戰
- 結論
第18章 全球人為因素與可用性工程服務市場
第19章 人為因素與可用性工程服務市場:依步驟
第20章 人為因素和可用性工程服務市場:依方法類型
第21章 人為因素和可用性工程服務市場:依測試的醫療器材類別
第22章 人為因素和可用性工程服務市場:依地區
第23章 人為因素和可用性工程服務市場:依主要參與者
第24章 結論
第25章 高階主管見解
- 章節概述
- ClariMed
- Loring Human Factors
- Cambridge Design Partnership
- Ergosign
- DCA
- Thay Medical
第26章 附錄 I:表格資料
第27章 附錄2:公司與組織名單
HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: OVERVIEW
As per Roots Analysis, the global human factors and usability engineering services market is estimated to grow from USD 0.94 billion in the current year to USD 1.97 billion by 2035, at a CAGR of 6.9% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Steps Involved
- Summative Study
- Contextual Analysis
- Formative Study
- Known Use Error Analysis
- Use Risk Analysis
- Submission Preparation
- Design Process
- Task Analysis
Type of Research Method
- Evaluation Research
- Generative Research
Class of Medical Device Tested
- Class I Medical Devices
- Class II Medical Devices
- Class III Medical Devices
Key Geographical Regions
- North America (US, Canada)
- Europe (Germany, France, Italy, Switzerland, UK, Rest of
the Europe)
- Asia-Pacific (China, Japan, Australia, India, Rest of Asia-Pacific)
- Middle East and North Africa Latin America
HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: GROWTH AND TRENDS
Over the years, human factors and usability engineering have become an integral part of the development of all classes of medical devices. As a result, the developers have actively started relying on the service providers for conducting human factors research for their devices (in development) in order to enhance device safety, efficacy and user experience. As medical devices become more complex, it is imperative to focus on the prototype and user interface design so as to minimize any potential risk associated with the use. Further, regulatory bodies (especially, the USFDA) are increasingly mandating the incorporation of human factors into the design and evaluation of medical devices. These bodies have started to employ a systematic method to assess manufacturers' adherence to usability engineering standards.
The emphasis on human factors and usability engineering in the medical device sector reflects a broader commitment to patient safety and device usability. This potentiates the need to conduct human factors and usability engineering on the devices throughout the product lifecycle (rather than conducting at later stages of development). This will support the device developers in timely facilitating the tweaks in the device development process so as to enable user satisfaction and eliminate risks. With the growing demand for innovative and personalized medical devices, the outsourcing of human factors and usability engineering services is anticipated to rise in the near future.
HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: KEY INSIGHTS
The report delves into the current state of the human factors and usability engineering services market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- Presently, over 210 companies claim to have the required expertise to offer human factors and usability engineering services for various medical devices; notably, most of the start-ups are based in Europe.

- More than 95% of the service providers offer product design and development related services; of these, majority of service providers support the testing of diagnostic devices.
- In order to gain an edge in this competitive industry, companies are continuously upgrading their capabilities to enhance their respective human factor engineering service portfolios.
- The rising interest in this domain is reflected by the wide array of mergers and acquisitions occurred in the recent past; in fact, close to 50% of the deals were inked in the last three years.
- Owing to the benefits offered by outsourcing human factors and usability engineering services, the market is anticipated to grow at an annualized rate of over 6.9% in the coming decade.

HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: KEY SEGMENTS
Currently, Summative Studies Account for the Largest Share of the Human Factors and Usability Engineering Services Market
Based on the steps involved, the market is segmented into summative study, contextual analysis, formative study, known use error analysis, use risk analysis, submission preparation, design process and task analysis. Currently, summative study holds the largest market share and is poised for the highest growth during the forecast period. This can be attributed to the fact that summative studies play a critical role in validating the safety and effectiveness of medical devices. Further, contextual analysis captures a significant share of the human factors and usability engineering services market. It is worth highlighting that contextual analysis offers various advantages, such as decreased chance of device recall, high return on investments (ROI) and expedition in the market launch of the medical device.
Evaluation Research is Likely to Dominate the Human Factors and Usability Engineering Services Market During the Forecast Period
Based on the type of research method, the market is segmented into evaluation research and generative research. At present, evaluation research segment occupies the highest market share of the human factors and usability engineering services market. This trend is unlikely to change in the near future.
Currently, Class III Medical Devices Testing Segment Occupies the Largest Share of the Human Factors and Usability Engineering Services Market
Based on the class of medical device tested, the market is segmented into class I, class II and class III. At present, class III medical device testing holds the maximum share of the human factors and usability engineering services market. This can be attributed to the fact that class III devices are considered high-risk devices and have the potential to cause serious illness or injury to the patients. Moreover, class II medical devices (including the IVD and injectors) require careful consideration of human factors engineering to ensure safe and effective use.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Middle East and North Africa, and Latin America. Majority share is expected to be captured by players based in North America and Europe. This can be attributed to the stringent regulatory guidelines for medical device approvals in this region. It is worth highlighting that, over the years, the market in the rest of the world is expected to grow at a higher CAGR.
Example Players in the Human Factors and Usability Engineering Services Market
- Aptar Digital Health
- AYES
- Bayoomed
- Bold Insight
- ClariMed
- Comate
- D+I
- Greenlight Guru
- Hydrix
- Invetech
- Johari Digital
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Chief Executive Officer and Chair, ClariMed
- Managing Director and Human Factors Consultant, THAY Medical
- Head of Human Factors, Cambridge Design Partnership
- Head of Research (Human Factors and Interaction), DCA
- UX Director and Team Manager, Ergosign
HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the human factors and usability engineering services market, focusing on key market segments, including [A] steps involved, [B] type of research method, [C] class of medical device tested, and [D] geographical regions.
- Market Landscape: A comprehensive evaluation of human factors and usability engineering service providers, considering various parameters, such as [A] year of establishment, [B] company size (in terms of employee count), [C] location of headquarters, [D] type of service offered, [E] concept phase, [F] design and development, [G] verification and validation testing, [H] type of medical devices tested, [I] medical device users and [J] software engineering offered.
- Regulatory Landscape Analysis: A discussion on general regulatory guidelines established and issued by major regulatory bodies for medical device approval. In addition, it features a brief description of human factors and usability engineering process followed by regulatory bodies in the US and the EU, their comparison and a common approach that can be adopted to satisfy guidelines issued by authorities of the aforementioned regions.
- Company Competitiveness Analysis: A comprehensive competitive analysis of service providers engaged in the human factors and usability engineering market, examining factors such as [A] company strength and [B] portfolio strength.
- Company Profiles: In-depth profiles of key human factors and usability engineering service providers based in North America, Europe and Asia-Pacific, focusing on [A] company overviews, [B] service portfolio, [C] recent developments and [D] an informed future outlook.
- Mergers and Acquisitions: A comprehensive examination of the various mergers and acquisitions, focusing on multiple relevant parameters, including [A] year of agreement, [B] type of agreement and [C] geographical location of the companies.
- Case Study: The chapter discusses medical device recalls, focusing on the yearly trends in recalls and the potential underlying causes. It also highlights the top five medical device recalls and suggests strategies for incorporating human factors and usability engineering into medical devices to minimize errors and decrease the frequency of recalls.
- Mega Trends: A qualitative assessment of the various megatrends ongoing in the human factors and usability engineering market.
- Additional Insight: A detailed discussion on the cost implications across various steps of the human factors and usability engineering process, namely contextual inquiry, task analysis, design process, formative study, use risk analysis, known use error analysis, summative study, submission preparation, and the cost requirements across each of the aforementioned stages.
- Market Impact Analysis: The report analyzes various factors such as drivers, restraints, opportunities, and challenges affecting the market growth.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Market Share Insights
- 1.3. Key Market Insights
- 1.4. Report Coverage
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.2.1. Market Landscape and Market Trends
- 2.2.2. Market Forecast and Opportunity Analysis
- 2.2.3. Comparative Analysis
- 2.3. Database Building
- 2.3.1. Data Collection
- 2.3.2. Data Validation
- 2.3.3. Data Analysis
- 2.4. Project Methodology
- 2.4.1. Secondary Research
- 2.4.1.1. Annual Reports
- 2.4.1.2. Academic Research Papers
- 2.4.1.3. Company Websites
- 2.4.1.4. Investor Presentations
- 2.4.1.5. Regulatory Filings
- 2.4.1.6. White Papers
- 2.4.1.7. Industry Publications
- 2.4.1.8. Conferences and Seminars
- 2.4.1.9. Government Portals
- 2.4.1.10. Media and Press Releases
- 2.4.1.11. Newsletters
- 2.4.1.12. Industry Databases
- 2.4.1.13. Roots Proprietary Databases
- 2.4.1.14. Paid Databases and Sources
- 2.4.1.15. Social Media Portals
- 2.4.1.16. Other Secondary Sources
- 2.4.2. Primary Research
- 2.4.2.1. Types of Primary Research
- 2.4.2.1.1. Qualitative Research
- 2.4.2.1.2. Quantitative Research
- 2.4.2.1.3. Hybrid Approach
- 2.4.2.2. Advantages of Primary Research
- 2.4.2.3. Techniques for Primary Research
- 2.4.2.3.1. Interviews
- 2.4.2.3.2. Surveys
- 2.4.2.3.3. Focus Groups
- 2.4.2.3.4. Observational Research
- 2.4.2.3.5. Social Media Interactions
- 2.4.2.4. Key Opinion Leaders Considered in Primary Research
- 2.4.2.4.1. Company Executives (CXOs)
- 2.4.2.4.2. Board of Directors
- 2.4.2.4.3. Company Presidents and Vice Presidents
- 2.4.2.4.4. Research and Development Heads
- 2.4.2.4.5. Technical Experts
- 2.4.2.4.6. Subject Matter Experts
- 2.4.2.4.7. Scientists
- 2.4.2.4.8. Doctors and Other Healthcare Providers
- 2.4.2.5. Ethics and Integrity
- 2.4.2.5.1. Research Ethics
- 2.4.2.5.2. Data Integrity
- 2.4.2.1. Types of Primary Research
- 2.4.3. Analytical Tools and Databases
- 2.4.1. Secondary Research
3. MARKET DYNAMICS
- 3.1. Chapter Overview
- 3.2. Forecast Methodology
- 3.2.1. Top-down Approach
- 3.2.2. Bottom-up Approach
- 3.2.3. Hybrid Approach
- 3.3. Market Assessment Framework
- 3.3.1. Total Addressable Market (TAM)
- 3.3.2. Serviceable Addressable Market (SAM)
- 3.3.3. Serviceable Obtainable Market (SOM)
- 3.3.4. Currently Acquired Market (CAM)
- 3.4. Forecasting Tools and Techniques
- 3.4.1. Qualitative Forecasting
- 3.4.2. Correlation
- 3.4.3. Regression
- 3.4.4. Extrapolation
- 3.4.5. Convergence
- 3.4.6. Sensitivity Analysis
- 3.4.7. Scenario Planning
- 3.4.8. Data Visualization
- 3.4.9. Time Series Analysis
- 3.4.10. Forecast Error Analysis
- 3.5. Key Considerations
- 3.5.1. Demographics
- 3.5.2. Government Regulations
- 3.5.3. Reimbursement Scenarios
- 3.5.4. Market Access
- 3.5.5. Supply Chain
- 3.5.6. Industry Consolidation
- 3.5.7. Pandemic / Unforeseen Disruptions Impact
- 3.6. Key Market Segmentation
- 3.7. Robust Quality Control
- 3.8. Limitations
4. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 4.1. Chapter Overview
- 4.2. Market Dynamics
- 4.2.1. Time Period
- 4.2.1.1. Historical Trends
- 4.2.1.2. Current and Future Estimates
- 4.2.2. Currency Coverage and Foreign Exchange Rate
- 4.2.2.1. Major Currencies Affecting the Market
- 4.2.2.2. Factors Affecting Currency Fluctuations and Foreign Exchange Rates
- 4.2.2.3. Impact of Foreign Exchange Rate Volatility on the Market
- 4.2.2.4. Strategies for Mitigating Foreign Exchange Risk
- 4.2.3. Trade Policies
- 4.2.3.1. Impact of Trade Barriers on the Market
- 4.2.3.2. Strategies for Mitigating the Risks Associated with Trade Barriers
- 4.2.4. Recession
- 4.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 4.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 4.2.5. Inflation
- 4.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 4.2.5.2. Potential Impact of Inflation on the Market Evolution
- 4.2.1. Time Period
5. EXECUTIVE SUMMARY
- 5.1. Chapter Overview
- 5.2. Executive Summary: Market Landscape
- 5.3. Executive Summary: Mergers and Acquisitions
- 5.4. Executive Summary: Market Forecast and Opportunity Analysis
6. INTRODUCTION
- 6.1. Chapter Overview
- 6.2. Overview of Human Factors and Usability Engineering
- 6.3. Human Factors Influencing Medical Device Design
- 6.3.1. End Users
- 6.3.2. User Environment
- 6.3.3. Device-User Interface
- 6.4. Key Steps Involved in Human Factors and Usability Engineering
- 6.5. Outsourcing of Human Factors and Usability Engineering
- 6.6. Advantages of Outsourcing Human Factors and Usability Engineering
- 6.7. Risks and Challenges Associated with Outsourcing Human Factors and Usability Engineering
- 6.8. Key Considerations while Selecting a Human Factors and Usability Engineering Service Provider
- 6.9. Conclusion
7. REGULATORY GUIDELINES FOR HUMAN FACTORS AND USABILITY ENGINEERING
- 7.1. Chapter Overview
- 7.2. Key Regulatory Authorities for Human Factors and Usability Engineering
- 7.3. Regulatory Landscape in North America
- 7.3.1. The USFDA Recognized Standards on Human Factors
and Usability Engineering
- 7.3.2. The USFDA Guidance Documents Related to Human Factors and Usability Engineering
- 7.3.3. Human Factors and Usability Engineering Process by the USFDA
- 7.4. Regulatory Landscape in Europe
- 7.4.1. EU Recognized Standards for Human Factors and Usability Engineering
- 7.4.2. Human Factors and Usability Engineering Process by European Union
- 7.5. Comparison between the USFDA and EU Guidelines
- 7.5.1. Common Approach to Make the USFDA and EU Submissions
- 7.5.2. Human Factors and Usability Engineering Process for Other Devices
- 7.6. Recent Activity of Regulatory Bodies Involved
- 7.7. Concluding Remarks
8. MARKET LANDSCAPE
- 8.1. Chapter Overview
- 8.2. Human Factors and Usability Engineering Service Providers: Overall Market Landscape
- 8.2.1. Analysis by Year of Establishment
- 8.2.2. Analysis by Company Size
- 8.2.3. Analysis by Location of Headquarters
- 8.2.4. Analysis by Company Size and Location of Headquarters
- 8.2.5. Analysis by Type of Service Offered
- 8.2.5.1. Analysis by Concept Phase
- 8.2.5.2. Analysis by Design and Development
- 8.2.5.3. Analysis by Verification and Validation
- 8.2.6. Analysis by Type of Medical Device Tested
- 8.2.7. Analysis by End User of Medical Device Tested
- 8.2.8. Analysis by Software
Engineering Offered
9. COMPANY COMPETITIVENSS ANALYSIS
- 9.1. Chapter Overview
- 9.2. Assumptions and Key Parameters
- 9.3. Methodology
- 9.4. Company Competitiveness Analysis: Human Factors and Usability Engineering Service Providers
- 9.4.1. Human Factors and Usability Engineering Service Providers based in North America (Peer Group I)
- 9.4.2. Human Factors and Usability Engineering Service Providers based in Europe (Peer Group II)
- 9.4.3. Human Factors and Usability Engineering Service Providers based in Asia-Pacific, Middle East and North Africa, and Latin America (Peer Group III)
10. COMPANY PROFILES: SERVICE PROVIDERS IN NORTH AMERICA
- 10.1. Chapter Overview
- 10.2. ClariMed
- 10.2.1. Company Overview
- 10.2.2. Service Portfolio
- 10.2.3. Recent Developments and Future Outlook
- 10.3. Aptar Digital Health
- 10.3.1. Company Overview
- 10.3.2. Service Portfolio
- 10.3.3. Recent Developments and Future Outlook
- 10.4. Bold Insight
- 10.4.1. Company Overview
- 10.4.2. Service Portfolio
- 10.4.3. Recent Developments and Future Outlook
- 10.5. Greenlight Guru
- 10.5.1. Company Overview
- 10.5.2. Service Portfolio
- 10.5.3. Recent Developments and Future Outlook
- 10.6. Key Winning Strategies
11. COMPANY PROFILES: SERVICE PROVIDERS IN EUROPE
- 11.1. Chapter Overview
- 11.2. Bayoomed
- 11.2.1. Company Overview
- 11.2.2. Service Portfolio
- 11.2.3. Recent Developments and Future Outlook
- 11.3. AYES
- 11.3.1. Company Overview
- 11.3.2. Service Portfolio
- 11.3.3. Recent Developments and Future Outlook
- 11.4. Comate
- 11.4.1. Company Overview
- 11.4.2. Service Portfolio
- 11.4.3. Recent Developments and Future Outlook
- 11.5. Key Winning Strategies
12. COMPANY PROFILES: SERVICE PROVIDERS IN ASIA-PACIFIC
- 12.1. Chapter Overview
- 12.2. Hydrix
- 12.2.1. Company Overview
- 12.2.2. Service Portfolio
- 12.2.3. Recent Developments and Future Outlook
- 12.3. D+I
- 12.3.1. Company Overview
- 12.3.2. Service Portfolio
- 12.3.3. Recent Developments and Future Outlook
- 12.4. Invetech
- 12.4.1. Company Overview
- 12.4.2. Service Portfolio
- 12.4.3. Recent Developments and Future Outlook
- 12.5. Johari Digital
- 12.5.1. Company Overview
- 12.5.2. Service Portfolio
- 12.5.3. Recent Developments and Future Outlook
- 12.6. Key Winning Strategies
13. MERGERS AND ACQUISITIONS
- 13.1. Chapter Overview
- 13.2. Type of Mergers and Acquisitions
- 13.3. Mergers and Acquisitions: Human Factors and Usability Engineering Service Providers
- 13.3.1. Analysis by Year of Agreement
- 13.3.2. Analysis by Type of Agreement
- 13.3.3. Analysis by Geography
- 13.3.3.1. Intracontinental and Intercontinental Agreements
- 13.3.3.2. International and Local Agreements
- 13.4. Most Active Players: Analysis by Number of Agreements
14. COST IMPLICATIONS RELATED TO HUMAN FACTORS AND USABILITY ENGINEERING PROCESS
- 14.1. Chapter Overview
- 14.2. Key Steps involved in Human Factors and Usability Engineering Process
- 14.3. Cost Distribution across the Different Steps of Human Factors and Usability Engineering
- 14.3.1. Costs Associated with Contextual Inquiry
- 14.3.2. Costs Associated with Task Analysis
- 14.3.3. Costs Associated with Design Analysis
- 14.3.4. Costs Associated with Formative Study
- 14.3.5. Costs Associated with Use Risk Analysis
- 14.3.6. Costs Associated with Known Use Error Analysis
- 14.3.7. Costs Associated with Summative Study
- 14.3.8. Costs Associated with Submission Preparation
15. CASE STUDY: MEDICAL DEVICE RECALLS AND ROLE OF HUMAN FACTORS AND USABILITY ENGINEERING
- 15.1. Chapter Overview
- 15.2. Medical Device Recalls
- 15.3. Major Medical Device Recalls
- 15.3.1. Philips: Recall of Sense XL Torso Coil
- 15.3.2. Inspire Medical Systems: Recall of Inspire IV Implantable Pulse Generator (IPG) Model 3028
- 15.3.3. Hamilton Medical: Recall of HAMILTON-C6 Intensive Care Ventilator
- 15.3.4. Zoll Medical: Recall of ZOLL 731 Ventilator
- 15.3.5. Elekta Instrument: Recall of Disposable Biopsy Needle Kit
- 15.4. Medical Device Recalls: Role Of Human Factors and Usability Engineering
- 15.5. Conclusion
16. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: ONGOING MEGATRENDS
- 16.1. Chapter Overview
- 16.2. Key Megatrends
17. MARKET IMPACT ANALYSIS
- 17.1. Chapter Overview
- 17.2. Market Drivers
- 17.3. Market Restraints
- 17.4. Market Opportunities
- 17.5. Market Challenges
- 17.6. Conclusion
18. GLOBAL HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET
- 18.1. Chapter Overview
- 18.2. Assumptions and Methodology
- 18.3. Global Human Factors and Usability Engineering Services Market, till 2035
- 18.3.1. Roots Analysis Perspective on Market Growth
- 18.3.2. Scenario Analysis
- 18.3.2.1. Conservative Scenario
- 18.3.2.2. Optimistic Scenario
- 18.4. Key Market Segmentations
19. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY STEPS INVOLVED
- 19.1. Chapter Overview
- 19.2. Assumptions and Methodology
- 19.3. Human Factors and Usability Engineering Services Market: Distribution by Steps Involved, Current Year and 2035
- 19.3.1. Human Factors and Usability Engineering Services Market for Summative Study, till 2035
- 19.3.2. Human Factors and Usability Engineering Services Market for Contextual Analysis, till 2035
- 19.3.3. Human Factors and Usability Engineering Services Market for Formative Study, till 2035
- 19.3.4. Human Factors and Usability Engineering Services Market for Known Use Error Analysis, till 2035
- 19.3.5. Human Factors and Usability Engineering Services Market for Use Risk Analysis, till 2035
- 19.3.6. Human Factors and Usability Engineering Services Market for Submission Preparation, till 2035
- 19.3.7. Human Factors and Usability Engineering Services Market for Design Analysis, till 2035
- 19.3.8. Human Factors and Usability Engineering Services Market for Task Analysis, till 2035
- 19.4. Penetration-Growth (P-G) Matrix
- 19.5. Data Triangulation and Validation
20. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY TYPE OF RESEARCH METHOD
- 20.1. Chapter Overview
- 20.2. Assumptions and Methodology
- 20.3. Human Factors and Usability Engineering Services Market: Distribution by Type of Research Method, Current Year and 2035
- 20.3.1. Human Factors and Usability Engineering Services Market for Evaluation Research, till 2035
- 20.3.2. Human Factors and Usability Engineering Services Market for Generative Research, till 2035
- 20.4. Penetration-Growth (P-G) Matrix
- 20.5. Data Triangulation and Validation
21. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY CLASS OF MEDICAL DEVICE TESTED
- 21.1. Chapter Overview
- 21.2. Assumptions and Methodology
- 21.3. Human Factors and Usability Engineering Services Market: Distribution by Class of Medical Device Tested, 2024 and 2035
- 21.3.1. Human Factors and Usability Engineering Services Market for Class I Medical Devices, till 2035
- 21.3.2. Human Factors and Usability Engineering Services Market for Class II Medical Devices, till 2035
- 21.3.3. Human Factors and Usability Engineering Services Market for Class III Medical Devices, till 2035
- 21.4. Penetration-Growth (P-G) Matrix
- 21.5. Data Triangulation and Validation
22. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY GEOGRAPHICAL REGIONS
- 22.1. Chapter Overview
- 22.2. Assumptions and Methodology
- 22.3. Human Factors and Usability Engineering Services Market: Distribution by Geographical Regions, Current Year and 2035
- 22.3.1. Human Factors and Usability Engineering Services Market in North America, till 2035
- 22.3.1.1. Human Factors and Usability Engineering Services Market in the US, till 2035
- 22.3.1.2. Human Factors and Usability Engineering Services Market in Canada, till 2035
- 22.3.2. Human Factors and Usability Engineering Services Market in Europe, till 2035
- 22.3.2.1. Human Factors and Usability Engineering Services Market in Germany, till 2035
- 22.3.2.2. Human Factors and Usability Engineering Services Market in France, till 2035
- 22.3.2.3. Human Factors and Usability Engineering Services Market in Italy, till 2035
- 22.3.2.4. Human Factors and Usability Engineering Services Market in the UK, till 2035
- 22.3.2.5. Human Factors and Usability Engineering Services Market in Switzerland, till 2035
- 22.3.2.6. Human Factors and Usability Engineering Services Market in Rest of Europe, till 2035
- 22.3.3. Human Factors and Usability Engineering Services Market in Asia-Pacific, till 2035
- 22.3.3.1. Human Factors and Usability Engineering Services Market in China, till 2035
- 22.3.3.2. Human Factors and Usability Engineering Services Market in Japan, till 2035
- 22.3.3.3. Human Factors and Usability Engineering Services Market in Australia, till 2035
- 22.3.3.4. Human Factors and Usability Engineering Services Market in India, till 2035
- 22.3.3.5. Human Factors and Usability Engineering Services Market in Rest of Asia-Pacific, till 2035
- 22.3.4. Human Factors and Usability Engineering Services Market in Middle East and North Africa, till 2035
- 22.3.5. Human Factors and Usability
- 22.3.1. Human Factors and Usability Engineering Services Market in North America, till 2035
Engineering Services Market in Latin America, till 2035
- 22.4. Market Movement Analysis
- 22.5. Penetration-Growth (P-G) Matrix
- 22.6. Data Triangulation and Validation
23. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY LEADING PLAYERS
- 23.1. Chapter Overview
- 23.2. Leading Industry Players
24. CONCLUSION
25. EXECUTIVE INSIGHTS
- 25.1. Chapter Overview
- 25.2. ClariMed
- 25.2.1. Company Snapshot
- 25.2.2. Interview Transcript: Chief Executive Officer and Chair
- 25.3. Loring Human Factors
- 25.3.1. Company Snapshot
- 25.3.2. Interview Transcript: Founder and Principal
- 25.4. Cambridge Design Partnership
- 25.4.1. Company Snapshot
- 25.4.2. Interview Transcript: Head of Human Factors
- 25.5. Ergosign
- 25.5.1. Company Snapshot
- 25.5.2. Interview Transcript: UX Director and Principal UX Designer
- 25.6. DCA
- 25.6.1. Company Snapshot
- 25.6.2. Interview Transcript: Head of Research (Human Factors and Interaction)
- 25.7. Thay Medical
- 25.7.1. Company Snapshot
- 25.7.2. Interview Transcript: Managing Director and Human Factors Consultant
26. APPENDIX I: TABULATED DATA
27. APPENDIX II: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 7.1 The USFDA Recognized Standards for Human Factors and Usability Engineering Services
- Table 8.1 Human Factors and Usability Engineering: List of Service Providers
- Table 8.2 Human Factors and Usability Engineering Service Providers: Information on Type of Service Offered
- Table 8.3 Human Factors and Usability Engineering Service Providers: Information on Concept Phase, and Design and Development
- Table 8.4 Human Factors and Usability Engineering Service Providers: Information on Verification and Validation
- Table 8.5 Human Factors and Usability Engineering Service Providers: Information on Type of Medical Device Tested
- Table 8.6 Human Factors and Usability Engineering Service Providers: Information on End User of Medical Device Tested
- Table 8.7 Human Factors and Usability Engineering Service Providers: Information on Software Engineering Offered
- Table 10.1 Human Factors and Usability Engineering Service Providers: List of Companies Profiled (North America)
- Table 10.2 ClariMed: Company Overview
- Table 10.3 ClariMed: Service Portfolio
- Table 10.4 ClariMed: Recent Developments and Future Outlook
- Table 10.5 Aptar Digital Health: Company Overview
- Table 10.6 Aptar Digital Health: Service Portfolio
- Table 10.7 Aptar Digital Health: Recent Developments and Future Outlook
- Table 10.8 Bold Insight: Company Overview
- Table 10.9 Bold Insight: Service Portfolio
- Table 10.10 Bold Insight: Recent Developments and Future Outlook
- Table 10.11 Greenlight Guru: Company Overview
- Table 10.12 Greenlight Guru: Service Portfolio
- Table 10.13 Greenlight Guru: Recent Developments and Future Outlook
- Table 11.1 Human Factors and Usability Engineering Service Providers: List of Companies Profiled (Europe)
- Table 11.2 Bayoomed: Company Overview
- Table 11.3 Bayoomed: Service Portfolio
- Table 11.4 Bayoomed: Recent Developments and Future Outlook
- Table 11.5 AYES: Company Overview
- Table 11.6 AYES: Service Portfolio
- Table 11.7 Comate: Company Overview
- Table 11.8 Comate: Service Portfolio
- Table 11.9 Comate: Recent Developments and Future Outlook
- Table 12.1 Human Factors and Usability Engineering Service Providers: List of Companies Profiled (Asia-Pacific)
- Table 12.2 Hydrix: Company Overview
- Table 12.3 Hydrix: Service Portfolio
- Table 12.4 Hydrix: Recent Developments and Future Outlook
- Table 12.5 D+I: Company Overview
- Table 12.6 D+I: Service Portfolio
- Table 12.7 D+I: Recent Developments and Future Outlook
- Table 12.8 Invetech: Company Overview
- Table 12.9 Invetech: Service Portfolio
- Table 12.10 Invetech: Recent Developments and Future Outlook
- Table 12.11 Johari Digital: Company Overview
- Table 12.12 Johari Digital: Service Portfolio
- Table 12.13 Johari Digital: Recent Developments and Future Outlook
- Table 13.1 Human Factors and Usability Engineering Service Providers: List of Mergers and Acquisitions, since 2019
- Table 15.1 Philips: Recall of Sense XL Torso Coil
- Table 15.2 Inspire Medical Systems: Recall of Inspire IV Implantable Pulse Generator Model 3028
- Table 15.3 Hamilton Medical: Recall of HAMILTON-C6 Intensive Care Ventilator
- Table 15.4 Zoll Medical: Recall of ZOLL 731 Ventilator
- Table 15.5 Elekta Instrument: Recall of Disposable Biopsy Needle Kit
- Table 15.6 Approached for Mitigating the Risks Associated with Use Errors
- Table 23.1 Leading Industry Players: Based on General Market Understanding
- Table 23.2 Leading Industry Players: Based on Number of Services Offered
- Table 23.3 Leading Industry Players: Based on Number of Mergers and Acquisitions
- Table 25.1 ClariMed: Company Overview
- Table 25.2 Loring Human Factors: Company Overview
- Table 25.3 Cambridge Design Partnership: Company Overview
- Table 25.4 Ergosign: Company Overview
- Table 25.5 DCA: Company Overview
- Table 25.6 Thay Medical: Company Overview
- Table 26.1 Human Factors and Usability Engineering Service Providers: Distribution by Year of Establishment
- Table 26.2 Human Factors and Usability Engineering Service Providers: Distribution by Company Size
- Table 26.3 Human Factors and Usability Engineering Service Providers: Distribution by Location of Headquarters
- Table 26.4 Human Factors and Usability Engineering Service Providers: Distribution by Company Size and Location of Headquarters
- Table 26.5 Human Factors and Usability Engineering Service Providers: Distribution by Type of Service Offered
- Table 26.6 Human Factors and Usability Engineering Service Providers: Distribution by Concept Phase
- Table 26.7 Human Factors and Usability Engineering Service Providers: Distribution by Design and Development
- Table 26.8 Human Factors and Usability Engineering Service Providers: Distribution by Verification and Validation
- Table 26.9 Human Factors and Usability Engineering Service Providers: Distribution by Type of Medical Device Tested
- Table 26.10 Human Factors and Usability Engineering Service Providers: Distribution by End User of Medical Device Tested
- Table 26.11 Human Factors and Usability Engineering Service Providers: Distribution by Software Engineering Offered
- Table 26.12 Mergers and Acquisitions: Cumulative Year-Wise Trend, since 2019
- Table 26.13 Mergers and Acquisitions: Distribution by Type of Agreement
- Table 26.14 Mergers and Acquisitions: Distribution by Year and Type of Agreement
- Table 26.15 Most Active Players: Analysis by Number of Agreements
- Table 26.16 Mergers and Acquisitions: Intracontinental Agreement and Intercontinental Agreement
- Table 26.17 Mergers and Acquisitions: International Agreements and Local Agreement
- Table 26.18 Global Human Factors and Usability Engineering Services Market, till 2035: Base Scenario (USD Billion)
- Table 26.19 Global Human Factors and Usability Engineering Services Market, till 2035: Conservative Scenario (USD Billion)
- Table 26.20 Global Human Factors and Usability Engineering Services Market, till 2035: Optimistic Scenario (USD Billion)
- Table 26.21 Human Factors and Usability Engineering Services Market: Distribution by Steps Involved, Current Year and 2035 (USD Billion)
- Table 26.22 Human Factors and Usability Engineering Services Market for Summative Study, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.23 Human Factors and Usability Engineering Services Market for Contextual Analysis, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.24 Human Factors and Usability Engineering Services Market for Formative Study, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.25 Human Factors and Usability Engineering Services Market for Known Use Error Analysis, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.26 Human Factors and Usability Engineering Services Market for Use Risk Analysis, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.27 Human Factors and Usability Engineering Services Market for Submission Preparation, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.28 Human Factors and Usability Engineering Services Market for Design Analysis, till 2035, Conservative, Base and Scenarios (USD Billion)
- Table 26.29 Human Factors and Usability Engineering Services Market for Task Analysis, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.30 Human Factors and Usability Engineering Services Market: Distribution by Type of Research Method, Current Year and 2035 (USD Billion)
- Table 26.31 Human Factors and Usability Engineering Services Market for Evaluation Research, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.32 Human Factors and Usability Engineering Services Market for Generative Research, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.33 Human Factors and Usability Engineering Services Market: Distribution by Class of Medical Device Tested, 2024 and 2035 (USD Billion)
- Table 26.34 Human Factors and Usability Engineering Services Market for Class I Medical Devices, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.35 Human Factors and Usability Engineering Services Market for Class II Medical Devices, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.36 Human Factors and Usability Engineering Services Market for Class III Medical Devices, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.37 Human Factors and Usability Engineering Services Market: Distribution by Geographical Regions, Current Year and 2035 (USD Billion)
- Table 26.38 Human Factors and Usability Engineering Services Market in North America, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.39 Human Factors and Usability Engineering Services Market in the US, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.40 Human Factors and Usability Engineering Services Market in Canada, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.41 Human Factors and Usability Engineering Services Market in Europe, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.42 Human Factors and Usability Engineering Services Market in Germany, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.43 Human Factors and Usability Engineering Services Market in France, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.44 Human Factors and Usability Engineering Services Market in Italy, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.45 Human Factors and Usability Engineering Services Market in the UK, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.46 Human Factors and Usability Engineering Services Market in Switzerland, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.47 Human Factors and Usability Engineering Services Market in Rest of Europe, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.48 Human Factors and Usability Engineering Services Market in Asia-Pacific, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.49 Human Factors and Usability Engineering Services Market in China, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.50 Human Factors and Usability Engineering Services Market in Japan, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.51 Human Factors and Usability Engineering Services Market in Australia, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.52 Human Factors and Usability Engineering Services Market in India, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.53 Human Factors and Usability Engineering Services Market in Rest of Asia-Pacific, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.54 Human Factors and Usability Engineering Services Market in Middle East and North Africa, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
- Table 26.55 Human Factors and Usability Engineering Services Market in Latin America, till 2035, Conservative, Base and Optimistic Scenarios (USD Billion)
List of Figures
- Figure 2.1 Research Methodology: Project Methodology
- Figure 2.2 Research Methodology: Data Sources for Secondary Research
- Figure 3.1 Market Dynamics: Forecast Methodology
- Figure 3.2 Market Dynamics: Key Market Segmentation
- Figure 3.3 Market Dynamics: Robust Quality Control
- Figure 4.1 Lessons Learnt from Past Recessions
- Figure 5.1 Executive Summary: Market Landscape
- Figure 5.2 Executive Summary: Mergers and Acquisitions
- Figure 5.3 Executive Summary: Market Forecast and Opportunity Analysis
- Figure 6.1 Role of Human Factors and Usability Engineering in Medical Device Development
- Figure 6.2 Human Factor Influencing Medical Device Design
- Figure 6.3 End user Related Factors for Designing Medical Devices for Users
- Figure 6.4 Basic Components of Device-User Interface
- Figure 6.5 Key Steps Involved in Human Factors and Usability Engineering
- Figure 7.1 Key Regulatory Authorities for Human Factors and Usability Engineering
- Figure 7.2 Human Factors and Usability Engineering Process by the USFDA
- Figure 7.3 Human Factors and Usability Engineering Process by the European Union
- Figure 7.4 Comparison between the USFDA and EU Guidelines
- Figure 7.5 Common Approach to Make the USFDA and EU Submissions
- Figure 7.6 Human Factors and Usability Engineering Process for Other Devices
- Figure 8.1 Human Factors and Usability Engineering Service Providers: Distribution by Year of Establishment
- Figure 8.2 Human Factors and Usability Engineering Service Providers: Distribution by Company Size
- Figure 8.3 Human Factors and Usability Engineering Service Providers: Distribution by Location of Headquarters
- Figure 8.4 Human Factors and Usability Engineering Service Providers: Distribution by Company Size and Location of Headquarters
- Figure 8.5 Human Factors and Usability Engineering Service Providers: Distribution by Type of Service Offered
- Figure 8.6 Human Factors and Usability Engineering Service Providers: Distribution by Concept Phase
- Figure 8.7 Human Factors and Usability Engineering Service Providers: Distribution by Design and Development
- Figure 8.8 Human Factors and Usability Engineering Service Providers: Distribution by Verification and Validation
- Figure 8.9 Human Factors and Usability Engineering Service Providers: Distribution by Type of Medical Device Tested
- Figure 8.10 Human Factors and Usability Engineering Service Providers: Distribution by End User of Medical Device Tested
- Figure 8.11 Human Factors and Usability Engineering Service Providers: Distribution by Software Engineering Offered
- Figure 9.1 Company Competitiveness Analysis: Human Factors and Usability Engineering Service Providers based in North America (Peer Group I)
- Figure 9.2 Company Competitiveness Analysis: Human Factors and Usability Engineering Service Providers based in Europe (Peer Group II)
- Figure 9.3 Company Competitiveness Analysis: Human Factors and Usability Engineering Service Providers based in Asia-Pacific, Middle East and North Africa, and Latin America (Peer Group III)
- Figure 10.1 Key Winning Strategies: Distribution of Prominent Players in North America
- Figure 11.1 Key Winning Strategies: Distribution of Prominent Players in Europe
- Figure 12.1 Key Winning Strategies: Distribution of Prominent Players in Asia-Pacific
- Figure 13.1 Mergers and Acquisitions: Cumulative Year-Wise Trend, since 2019
- Figure 13.2 Mergers and Acquisitions: Distribution by Type of Agreement
- Figure 13.3 Mergers and Acquisitions: Distribution by Year and Type of Agreement
- Figure 13.4 Mergers and Acquisitions: Intracontinental and Intercontinental Agreements
- Figure 13.5 Mergers and Acquisitions: International and Local Agreements
- Figure 13.6 Most Active Players: Analysis by Number of Agreements
- Figure 14.1 Key Steps Involved in Human Factors and Usability Engineering
- Figure 14.2 Cost Distribution Across the Different Steps of Human Factors and Usability Engineering
- Figure 14.3 Costs Associated with Contextual Inquiry
- Figure 14.4 Costs Associated with Task Analysis
- Figure 14.5 Costs Associated with Design Analysis
- Figure 14.6 Costs Associated with Formative Study
- Figure 14.7 Costs Associated with Use Risk Analysis
- Figure 14.8 Costs Associated with Known Use Error Analysis
- Figure 14.9 Costs Associated with Summative Study
- Figure 14.10 Costs Associated with Submission Preparation
- Figure 15.1 Types of Medical Devices Use Errors
- Figure 16.1 Key Megatrends
- Figure 18.1 Global Human Factors and Usability Engineering Services Market, till 2035 (USD Billion)
- Figure 18.2 Global Human Factors and Usability Engineering Services Market, till 2035: Conservative Scenario (USD Billion)
- Figure 18.3 Global Human Factors and Usability Engineering Services Market, till 2035: Optimistic Scenario (USD Billion)
- Figure 19.1 Human Factors and Usability Engineering Services Market: Distribution by Steps Involved, Current Year and 2035
- Figure 19.2 Human Factors and Usability Engineering Services Market for Summative Study, till 2035 (USD Billion)
- Figure 19.3 Human Factors and Usability Engineering Services Market for Contextual Analysis, till 2035 (USD Billion)
- Figure 19.4 Human Factors and Usability Engineering Services Market for Formative Study, till 2035 (USD Billion)
- Figure 19.5 Human Factors and Usability Engineering Services Market for Known Use Error Analysis, till 2035 (USD Billion)
- Figure 19.6 Human Factors and Usability Engineering Services Market for Use Risk Analysis, till 2035 (USD Billion)
- Figure 19.7 Human Factors and Usability Engineering Services Market for Submission Preparation, till 2035 (USD Billion)
- Figure 19.8 Human Factors and Usability Engineering Services Market for Design Analysis, till 2035 (USD Billion)
- Figure 19.9 Human Factors and Usability Engineering Services Market for Task Analysis, till 2035 (USD Billion)
- Figure 19.10 Penetration-Growth (P-G) Matrix: Steps Involved
- Figure 20.1 Human Factors and Usability Engineering Services Market: Distribution by Type of Research Method, Current Year and 2035
- Figure 20.2 Human Factors and Usability Engineering Services Market for Evaluation Research, till 2035 (USD Billion)
- Figure 20.3 Human Factors and Usability Engineering Services Market for Generative Research, till 2035 (USD Billion)
- Figure 20.4 Penetration-Growth (P-G) Matrix: Type of Research Method
- Figure 21.1 Human Factors and Usability Engineering Services Market: Distribution by Class of Medical Device Tested, Current Year and 2035
- Figure 21.2 Human Factors and Usability Engineering Services Market for Class I Medical Devices, till 2035 (USD Billion)
- Figure 21.3 Human Factors and Usability Engineering Services Market for Class II Medical Devices, till 2035 (USD Billion)
- Figure 21.4 Human Factors and Usability Engineering Services Market for Class III Medical Devices, till 2035 (USD Billion)
- Figure 21.5 Penetration-Growth (P-G) Matrix: Class of Medical Device Tested
- Figure 22.1 Human Factors and Usability Engineering Services Market: Distribution by Geographical Regions, Current Year and 2035
- Figure 22.2 Human Factors and Usability Engineering Services Market in North America, till 2035 (USD Billion)
- Figure 22.3 Human Factors and Usability Engineering Services Market in the US, till 2035 (USD Billion)
- Figure 22.4 Human Factors and Usability Engineering Services Market in Canada, till 2035 (USD Billion)
- Figure 22.5 Human Factors and Usability Engineering Services Market in Europe, till 2035 (USD Billion)
- Figure 22.6 Human Factors and Usability Engineering Services Market in Germany, till 2035 (USD Billion)
- Figure 22.7 Human Factors and Usability Engineering Services Market in France, till 2035 (USD Billion)
- Figure 22.8 Human Factors and Usability Engineering Services Market in Italy, till 2035 (USD Billion)
- Figure 22.9 Human Factors and Usability Engineering Services Market in the UK, till 2035 (USD Billion)
- Figure 22.10 Human Factors and Usability Engineering Services Market in Switzerland, till 2035 (USD Billion)
- Figure 22.11 Human Factors and Usability Engineering Services Market in Rest of Europe, till 2035 (USD Billion)
- Figure 22.12 Human Factors and Usability Engineering Services Market in Asia-Pacific, till 2035 (USD Billion)
- Figure 22.13 Human Factors and Usability Engineering Services Market in China, till 2035 (USD Billion)
- Figure 22.14 Human Factors and Usability Engineering Services Market in Japan, till 2035 (USD Billion)
- Figure 22.15 Human Factors and Usability Engineering Services Market in Australia, till 2035 (USD Billion)
- Figure 22.16 Human Factors and Usability Engineering Services Market in India, till 2035 (USD Billion)
- Figure 22.17 Human Factors and Usability Engineering Services Market in Rest of Asia-Pacific, till 2035 (USD Billion)
- Figure 22.18 Human Factors and Usability Engineering Services Market in Middle East and North Africa, till 2035 (USD Billion)
- Figure 22.19 Human Factors and Usability Engineering Services Market in Latin America, till 2035 (USD Billion)
- Figure 22.20 Market Movement Analysis
- Figure 22.21 Penetration-Growth (P-G) Matrix: Geographical Regions
- Figure 24.1 Conclusion: Human Factors and Usability Engineering Services Providers Market Landscape
- Figure 24.2 Conclusion: Mergers and Acquisitions
- Figure 24.3 Conclusion: Market Forecast and Opportunity Analysis













