 |
市場調查報告書
商品編碼
1708203
生物標記市場機會、成長動力、產業趨勢分析及 2025 - 2034 年預測Biomarkers Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球生物標記市場價值為 776 億美元,預計將從 2025 年的 859 億美元成長到 2034 年的 2,448 億美元,複合年成長率為 12.3%。生物標記包括血液、組織和體液中的蛋白質、基因和其他分子,在識別生物過程和疾病狀況方面發揮著至關重要的作用。它們在早期疾病檢測、監測治療反應和開發個人化療法方面的應用繼續推動市場成長。個人化醫療的進步正在改變生物標記的檢測和治療方法,液體活體組織切片等創新技術可以實現非侵入性識別和即時監測治療反應。這些進步擴大了生物標記的臨床應用,使其成為改善患者預後和加速藥物發現的不可或缺的工具。
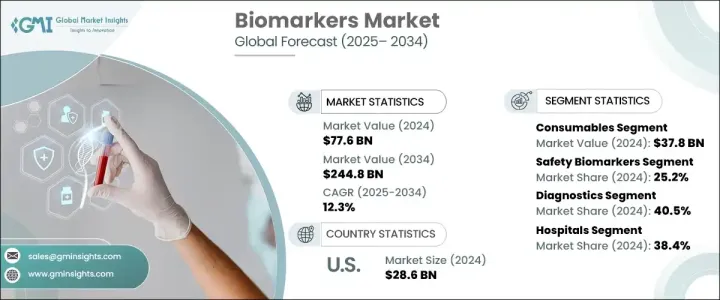
耗材部分包括試劑、檢測試劑盒和微孔板,2024 年創收 378 億美元,預計預測期內複合年成長率為 12.1%。在嚴格的品質控制標準下生產的耗材可確保生物標記研究(特別是在臨床診斷和藥物發現)的結果一致且可重複。它們與質譜、次世代定序 (NGS) 和液相層析等先進平台的兼容性提高了生物標記發現和分析的準確性和效率,從而推動了該領域的需求。
| 市場範圍 | |
|---|---|
| 起始年份 | 2024 |
| 預測年份 | 2025-2034 |
| 起始值 | 776億美元 |
| 預測值 | 2448億美元 |
| 複合年成長率 | 12.3% |
安全生物標記領域在 2024 年佔據了 25.2% 的市場佔有率,預計到 2034 年將達到 632 億美元。安全生物標記在早期檢測藥物或治療的潛在副作用、預防嚴重併發症以及最大限度地降低與後期臨床試驗失敗相關的成本方面發揮關鍵作用。這些生物標記有助於識別多個器官系統的毒性,從而可以持續監測器官功能並提高藥物開發的整體安全性。
診斷領域在 2024 年佔據了 40.5% 的市場佔有率,這得益於生物標記在早期識別疾病並透過及時干預改善預後的能力。非侵入性和微創生物標記檢測方法,包括血液、尿液和唾液測試,可提高患者的依從性,同時減少與傳統診斷方法相關的不適。下一代定序 (NGS) 和質譜等診斷技術的創新進一步提高了生物標記檢測的敏感度和準確性,擴大了其臨床實用性並促進了市場成長。
癌症領域仍是生物標記的主要應用領域,到 2024 年其市佔率將達到 38.5%。生物標記能夠早期發現各種癌症,提高成功治療的可能性,並區分良性和惡性腫瘤。它們還促進治療後監測,以識別潛在的癌症復發,確保及時介入並有助於改善患者的治療效果。
醫院在 2024 年佔據了 38.4% 的市場佔有率,由於其能夠管理大量慢性病患者並為先進的診斷程序提供高技能人員,因此繼續引領生物標記市場。對液體活體組織切片和伴隨診斷等非侵入性生物標記測試的需求不斷成長,進一步加強了醫院在生物標記領域的作用。
2024 年,北美的生物標記市場規模為 314 億美元,預計到 2034 年將達到 975 億美元,其中美國將以 286 億美元的規模位居榜首。心血管疾病、神經系統疾病和癌症等慢性疾病的發生率不斷上升,推動了美國生物標記市場的成長。這些疾病負擔的加重促使製藥公司、醫院和研究機構投資開發先進的診斷解決方案和創新療法,從而確保了生物標記市場的持續擴張。
目錄
第1章:方法論與範圍
第2章:執行摘要
第3章:行業洞察
- 產業生態系統分析
- 產業衝擊力
- 成長動力
- 慢性病盛行率上升
- 基因組學和蛋白質組學技術的進展
- 個人化醫療需求不斷成長
- 增加研發活動
- 產業陷阱與挑戰
- 生物標記開發和測試成本高昂
- 缺乏標準化
- 成長動力
- 成長潛力分析
- 監管格局
- 技術格局
- 未來市場趨勢
- 波特的分析
- PESTEL分析
第4章:競爭格局
- 介紹
- 公司市佔率分析
- 公司矩陣分析
- 主要市場參與者的競爭分析
- 競爭定位矩陣
- 策略儀表板
第5章:市場估計與預測:按產品和服務,2021 年至 2034 年
- 主要趨勢
- 耗材
- 軟體
- 服務
第6章:市場估計與預測:依生物標記類型,2021 年至 2034 年
- 主要趨勢
- 安全生物標記
- 療效生物標記
- 預測性生物標記
- 替代生物標記
- 藥效學生物標記
- 預後生物標記
- 驗證生物標記
第7章:市場估計與預測:按應用,2021 年至 2034 年
- 主要趨勢
- 診斷
- 藥物發現與開發
- 個人化醫療
- 疾病風險評估
- 其他應用
第8章:市場估計與預測:依疾病類型,2021 年至 2034 年
- 主要趨勢
- 癌症
- 心血管疾病
- 神經系統疾病
- 免疫疾病
- 其他疾病類型
第9章:市場估計與預測:依最終用途,2021 年至 2034 年
- 主要趨勢
- 醫院
- 診斷實驗室
- 學術和研究機構
- 其他最終用途
第10章:市場估計與預測:按地區,2021 年至 2034 年
- 主要趨勢
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 法國
- 西班牙
- 義大利
- 荷蘭
- 亞太地區
- 中國
- 日本
- 印度
- 澳洲
- 韓國
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中東和非洲
- 南非
- 沙烏地阿拉伯
- 阿拉伯聯合大公國
第 11 章:公司簡介
- Abbott Laboratories
- Agilent Technologies
- Bio-Rad Laboratories
- Becton, Dickinson and Company
- Eurofins Scientific
- Epigenomics
- F. Hoffmann-La Roche
- GE Healthcare
- Illumina
- Johnson & Johnson
- Merck KGaA
- PerkinElmer
- QIAGEN
- Siemens Healthineers
- Thermo Fisher Scientific
The Global Biomarkers Market was valued at USD 77.6 billion in 2024 and is projected to grow from USD 85.9 billion in 2025 to USD 244.8 billion by 2034 at a CAGR of 12.3%. Biomarkers, which include proteins, genes, and other molecules found in blood, tissues, and body fluids, play a crucial role in identifying biological processes and disease conditions. Their use in early disease detection, monitoring treatment response, and developing personalized therapies continues to drive market growth. Advances in personalized medicine are transforming biomarker detection and treatment approaches, with innovations such as liquid biopsies enabling non-invasive identification and real-time monitoring of treatment responses. These advancements have expanded the clinical applications of biomarkers, making them indispensable tools in improving patient outcomes and accelerating drug discovery.

The consumables segment, which includes reagents, assay kits, and microplates, generated USD 37.8 billion in 2024 and is projected to grow at a CAGR of 12.1% during the forecast period. Consumables manufactured under stringent quality control standards ensure consistent and reproducible results across biomarker studies, particularly in clinical diagnostics and drug discovery. Their compatibility with advanced platforms such as mass spectrometry, next-generation sequencing (NGS), and liquid chromatography enhances the accuracy and efficiency of biomarker discovery and analysis, driving demand in this segment.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $77.6 Billion |
| Forecast Value | $244.8 Billion |
| CAGR | 12.3% |
The safety biomarkers segment captured a 25.2% market share in 2024 and is expected to reach USD 63.2 billion by 2034. Safety biomarkers play a critical role in detecting potential adverse effects of drugs or treatments at an early stage, preventing severe complications, and minimizing costs associated with late-stage clinical trial failures. These biomarkers facilitate the identification of toxicity across multiple organ systems, allowing continuous monitoring of organ function and improving overall safety in drug development.
The diagnostics segment held a 40.5% market share in 2024, driven by the ability of biomarkers to identify diseases at an early stage and improve prognosis through timely intervention. Non-invasive and minimally invasive biomarker detection methods, including blood, urine, and saliva tests, enhance patient compliance while reducing discomfort associated with traditional diagnostic methods. Innovations in diagnostic technologies, such as NGS and mass spectrometry, further improve the sensitivity and accuracy of biomarker detection, expanding their clinical utility and boosting market growth.
The cancer segment, with a 38.5% market share in 2024, remains a dominant application of biomarkers. Biomarkers enable the early detection of various cancers, improving the likelihood of successful treatment and distinguishing between benign and malignant tumors. They also facilitate post-treatment surveillance to identify potential cancer recurrence, ensuring timely intervention and contributing to better patient outcomes.
Hospitals, which accounted for a 38.4% market share in 2024, continue to lead the biomarkers market due to their ability to manage a large volume of chronic disease patients and provide highly skilled personnel for advanced diagnostic procedures. The growing demand for non-invasive biomarker tests, such as liquid biopsy and companion diagnostics, has further strengthened the role of hospitals in the biomarker landscape.
North America generated USD 31.4 billion in 2024 and is expected to reach USD 97.5 billion by 2034, with the U.S. dominating the region at USD 28.6 billion in 2024. The rising prevalence of chronic diseases, including cardiovascular diseases, neurological disorders, and cancer, drives market growth in the U.S. The increasing burden of these diseases has prompted pharmaceutical companies, hospitals, and research institutions to invest in the development of advanced diagnostic solutions and innovative therapies, ensuring the continued expansion of the biomarkers market.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of chronic diseases
- 3.2.1.2 Advancements in genomic and proteomic technologies
- 3.2.1.3 Rising demand for personalized medicine
- 3.2.1.4 Increasing research and development activities
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High costs of biomarker development and testing
- 3.2.2.2 Lack of standardization
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product and Services, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Consumables
- 5.3 Software
- 5.4 Services
Chapter 6 Market Estimates and Forecast, By Biomarker Type, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Safety biomarkers
- 6.3 Efficacy biomarkers
- 6.4 Predictive biomarkers
- 6.5 Surrogate biomarkers
- 6.6 Pharmacodynamic biomarkers
- 6.7 Prognostics biomarkers
- 6.8 Validation biomarkers
Chapter 7 Market Estimates and Forecast, By Application, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Diagnostics
- 7.3 Drug discovery and development
- 7.4 Personalized medicine
- 7.5 Disease risk assessment
- 7.6 Other applications
Chapter 8 Market Estimates and Forecast, By Disease Type, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Cancer
- 8.3 Cardiovascular diseases
- 8.4 Neurological diseases
- 8.5 Immunological diseases
- 8.6 Other diseases types
Chapter 9 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Hospitals
- 9.3 Diagnostic laboratories
- 9.4 Academic and research institutions
- 9.5 Other end use
Chapter 10 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.3 Europe
- 10.3.1 Germany
- 10.3.2 UK
- 10.3.3 France
- 10.3.4 Spain
- 10.3.5 Italy
- 10.3.6 Netherlands
- 10.4 Asia Pacific
- 10.4.1 China
- 10.4.2 Japan
- 10.4.3 India
- 10.4.4 Australia
- 10.4.5 South Korea
- 10.5 Latin America
- 10.5.1 Brazil
- 10.5.2 Mexico
- 10.5.3 Argentina
- 10.6 Middle East and Africa
- 10.6.1 South Africa
- 10.6.2 Saudi Arabia
- 10.6.3 UAE
Chapter 11 Company Profiles
- 11.1 Abbott Laboratories
- 11.2 Agilent Technologies
- 11.3 Bio-Rad Laboratories
- 11.4 Becton, Dickinson and Company
- 11.5 Eurofins Scientific
- 11.6 Epigenomics
- 11.7 F. Hoffmann-La Roche
- 11.8 GE Healthcare
- 11.9 Illumina
- 11.10 Johnson & Johnson
- 11.11 Merck KGaA
- 11.12 PerkinElmer
- 11.13 QIAGEN
- 11.14 Siemens Healthineers
- 11.15 Thermo Fisher Scientific





